Petroleum and Crude Oil SS3 Chemistry Lesson Note
Download Lesson NoteTopic: Petroleum and Crude Oil
SPECIFIC OBJECTIVES: At the end of the lesson, pupils should be able to
- Discuss the origin, occurrence, and composition of petroleum in Nigeria
- Highlights the factors that affect the location of refineries in Nigeria
- Explain the fractional distillation of petroleum and major products
INSTRUCTIONAL TECHNIQUES:
- Identification,
- explanation,
- questions and answers,
- demonstration,
- videos from source
INSTRUCTIONAL MATERIALS:
- Videos,
- loudspeaker,
- textbook,
- pictures
NOTE
PETROLEUM
Crude oil or Petroleum is the chief source of hydrocarbons. Petroleum which means rock oil in Latin occurs as a dark, sticky, viscous liquid. It is found in huge underground deposits in many parts of the world. Natural gas is usually found together with it. Petroleum is a mixture of gaseous liquid and solid alkanes, alkenes, cycloalkanes, aromatic hydrocarbons, and others. Natural gas consists mainly of methane. Crude oil is a mixture of hydrocarbons. It exists in the liquid phase in natural underground reservoirs and remains liquid at atmospheric pressure after passing through surface-separating facilities.
Crude oil occurs in large quantities in Nigeria, especially in Bayelsa, Edo, Imo, Rivers, Delta, Abia, Ondo, and Cross River states. It is dark brown though its composition and consistency vary from place to place. Different oil-producing areas yield significantly different varieties of crude oil. We have light and heavy crude oil. The light one has low metal and sulphur content is light in colour, and flows easily. It is very expensive. The heavy one has high metal and sulphur content and must be heated to become fluid. Petroleum is a naturally occurring, yellow-to-black liquid found in geological formations beneath the Earth’s surface, which is commonly refined into various types of fuels. Components of petroleum are separated using a technique called fractional distillation.
It consists of hydrocarbons of various molecular weights and other organic compounds
ORIGIN OF CRUDE OIL AND NATURAL GAS
They are formed from the remains of marine algae and animals. When these tiny aquatic organisms died, their remains gradually settled on the seabeds. Over the years, the remains became covered by mud, silt, and other sediments. As the sediments piled up, their mass exerted great pressure on the lower layers, changing them to hard sedimentary rocks. During this process, bacterial activity, heat, and pressure probably changed the plant and animal remains into crude oil and natural gas.
Refining of Crude Oil
Petroleum or crude oil occurs naturally. It contains many useful products also called fractions. These are separated by the method of fractional distillation. This process of obtaining useful fractions from petroleum is called refining.
The process of dividing petroleum into fractions with different boiling range volatilities and free from impurities is called refining.
The process of turning petroleum into a useful form is done in a crude oil refinery. The steps for making crude oil into oil, petrol, or whatever are fractional distillation, cracking, and reforming.
Petroleum is refined by fractional distillation. The process of separating a mixture into a series of fractions of different volatilities through distillation is known as fractional distillation.
In the process of fractional distillation, a mixture of different liquids is evaporated followed by condensation. Different liquids are evaporated according to their boiling point and they are collected in different chambers of the distillation tower
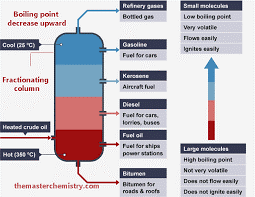
Fractional Distillation of Crude Oil
Fractional distillation differs from distillation only in that it separates a mixture into several different parts, called fractions. A tall column is fitted above the mixture, with several condensers coming off at different heights. The column is hot at the bottom and cool at the top. Substances with high boiling points condense at the bottom and substances with low boiling points condense at the top. Like distillation, fractional distillation works because the different substances in the mixture have different boiling points.
Fractions of petroleum from refining are petroleum gases (Methane, butane, etc.), petrol or gasoline, kerosene, diesel, lubricating oil, and bitumen (asphalt). Because they have different boiling points, the substances in crude oil can be separated using fractional distillation. The crude oil is evaporated and its vapours are allowed to condense at different temperatures in the fractionating column. Each fraction contains hydrocarbon molecules with a similar number of carbon atoms.
The mixture is inserted at the bottom, where mostly everything will condense as the temperature is 350°C and more. The condensed crude oil will rise to the next fraction above, which has a very high temperature as well, but a slightly smaller one. Only the part of the mixture, whose boiling point is under the temperature of the fraction, will condense and rise to the next fraction. The part of the mixture, whose boiling point is higher than the temperature inside the distillation fraction, will stay there and be pumped out.
Crude oil is heated until it boils and then the hydrocarbon gases are entered into the bottom of the fractionating column. As the gases go up the column the temperature decreases.
The hydrocarbon gases condense back into liquids and the fractions are removed from the sides of the column. The different fractions have different uses. The smaller the hydrocarbon molecule, the further it rises the column before condensing.
The fractionating column operates continuously. The temperatures shown are approximate. A sample of crude oil may be separated in the laboratory by fractional distillation. The collection vessel is changed as the temperature rises to collect the different fractions.
PETROLEUM FRACTIONS AND THEIR USES
EVALUATION:
- Discuss the origin, occurrence, and composition of petroleum in Nigeria
- Highlights the factors that affect the location of refineries in Nigeria
- Explain the fractional distillation of petroleum and major products
NOTE
Cracking
After the fractional distillation process, the separated mixtures have to be cracked down. This means that a long molecule will be split up into smaller parts.
Firstly, single bonds will be broken down. This results in some lone electrons.
The lone electrons form double bonds. Thus, hydrogen will disconnect from the carbon atom. Hydrogen (H2) remains as a side product. The loss of hydrogen in these smaller organic molecules is logical because when they are lost, more lone electrons remain with what the previous lone electrons can make a bond.
Reforming
After cracking, the molecules are ready to undergo the reforming process.
This is given by the octane number. The octane number is very important in petrol. It tells what the percentage of pure heptane (in the earlier days it was octane – that is why it is called octane number) in petrol is. This is of great importance for chemical behaviour. The quality of petrol is improved by adding mixtures to pure heptane. The chains of heptane are heated up (where platinum is used as a catalyst). So they can change. After the heating process, it shows a higher amount of branched chains. This increases the octane number.
The petrochemical industry
About 90% of the crude oil produced is used as a fuel to generate electricity and drive motor vehicles. Another 10% is used as petrochemical feedstock.
USES OF CRUDE OIL
- Used As petrochemical feedstock
- A source of hydrocarbons (e.g. methane, ethane, propane etc.). Used for manufacturing:
a. polymers
b.fertilisers and pesticides
c. cosmetics
d. solvents -dyes
3. Used for the synthesis of other organic compounds (alcohols, alkanoic acids, ethers, aldehydes, amines etc.)
NATURAL GAS
Natural gas is usually found together with crude oil in between rock layers; it is predominantly methane (about 90%). Other gases present include ethane propane, butane pentane, and a small fraction of carbon dioxide, nitrogen, and helium
Packaging as liquefied natural gas (LNG)
Liquefied natural gas (LNG) is natural gas (predominantly methane, CH4, with some mixture of ethane, C2H6) that has been cooled down to liquid form for ease and safety of non-pressurized storage or transport. It takes up about 1/600th of the volume of natural gas in the gaseous state.
It is odourless, colourless, non-toxic, and non-corrosive. Hazards include flammability after vaporisation into a gaseous state, freezing, and asphyxia.
The liquefaction process involves the removal of certain components, such as dust, acid gases, helium, water, and heavy hydrocarbons, which could cause difficulty downstream. The natural gas is then condensed into a liquid at close

USES OF NATURAL GAS
Natural gas is a non-renewable hydrocarbon used as a source of energy for heating, cooking, and electricity generation. It is also used as a fuel for vehicles and as a chemical feedstock in the manufacture of plastics and other commercially important organic chemicals.
EVALUATION:
- Explain the terms cracking and reforming
- Highlights three uses of crude oil
- Describe Natural gas
- What are the uses of natural gas?
CLASSWORK: As in evaluation
CONCLUSION: The teacher commends the students positively