Models of an Atom SS3 Physics Lesson Note
Download Lesson NoteTopic: Models of an Atom
SPECIFIC OBJECTIVES: At the end of the lesson the students should be able to;
- State and discuss what chemical evidence there is for the existence of atoms.
- Explain what experimental evidence there is for believing that matter is electrical.
- Explain the Bohr-Rutherford model of an atom.
INSTRUCTIONAL RESOURCES: A chart showing the diagram of an atom.
MODELS OF THE ATOM
Over the years, scientists have developed many different models of the atom. Each model has helped us to better understand the structure and behaviour of atoms. Atoms are tiny particles that makeup matter. They are so small that we cannot see them without the help of a powerful microscope. An atom is estimated to have a diameter of 10-10 m. To understand the nature of an atom, scientists use mathematics and models of atoms. The various models of atoms are as follows:
- Dalton’s Atomic model
- The plum pudding model was proposed by J.J Thomson.
- Planetary or nuclear model proposed by Ernest Rutherford.
- Bohr’s Atomic Model.
- Electron cloud atomic model.
- Dalton’s atomic model (1803) was the first model of the atom. Dalton proposed that atoms are indivisible and that they are like the building blocks of matter.

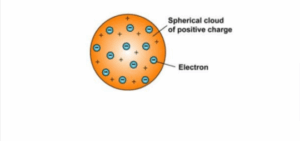
Thomson model The first model of the atom was proposed by J. J. Thomson in 1904. This model, known as the plum pudding model, depicts the atom as a sphere of positive charge with electrons embedded in it.
Rutherford Model In 1911, Ernest Rutherford proposed a new model of the atom, known as the nuclear model. This model depicted the atom as having a small, dense nucleus surrounded by electrons, that the atom is mostly space, with a small, dense nucleus at the centre. The nucleus contains the protons and neutrons, while the electrons orbit the nucleus.

APPLICATION: The teacher then guides students to mention the application of the photoelectric effects such as
- They are used in line spectra
- They are used in the study of the atom
EVALUATION: Mode: whole class Teacher’s Role: Ask the students to solve the following questions
- Explain J . J . Thomson’s atomic model
- What is Thomson’s atomic success?
Student’s Role: Answer the questions.
ASSIGNMENT: Study the Nucleus for the next class.
REFERENCE MATERIAL:
- New system physics for secondary school by Dr. Charles Chew et al
- New school physics by MW Anyakoha
- Internet facility.