Polymers, Giants And Macro Molecules SS3 Chemistry Lesson Note
Download Lesson NoteTopic: Polymers, Giants And Macro Molecules
SPECIFIC OBJECTIVES: At the end of the lesson, pupils should be able to
- Identify sources of sugar
- Identify and explain the classes of sugar
- Describe sugar as a reducing agent and also as a non-reducing agent
INSTRUCTIONAL TECHNIQUES:
- Identification,
- explanation,
- questions and answers,
- demonstration,
- videos from source
INSTRUCTIONAL MATERIALS:
- Videos,
- loudspeaker,
- textbook,
- pictures
NOTE
Sources of Sugar
Sugar can be found in various foods and drinks. Common sources include fruits, sugary snacks, desserts, soft drinks, and sweetened beverages. It’s also naturally present in some vegetables and dairy products..
Classification of sugar
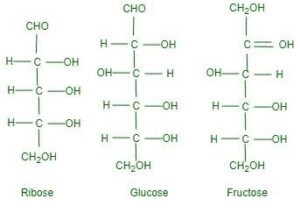
- Monosaccharides, the simplest form of carbohydrates, can be classified based on the number of carbon atoms in their structure. Common classifications include trioses (3 carbons), tetroses (4 carbons), pentoses (5 carbons), and hexoses (6 carbons). Examples of hexoses, which are crucial include glucose and fructose.
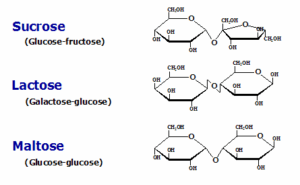
2. Disaccharides are sugars composed of two monosaccharide units. The following are some common disaccharides:
i. Sucrose:
– Composition: Glucose + Fructose
– Source: Table sugar, sugar beets, sugar cane
ii. Lactose:
– Composition: Glucose + Galactose
– Source: Milk and dairy products
iii. Maltose:
– Composition: Glucose + Glucose
– Source: Found in germinating grains and malt products.
NOTE: Each disaccharide consists of a unique combination of monosaccharide units, contributing to different tastes and properties.
- Polysaccharides are complex sugars composed of multiple monosaccharide units. Below are some common polysaccharides:
i. Starch:
– Composed of Glucose units
– Source: Main energy storage in plants; found in grains, potatoes, and root vegetables.
ii. Glycogen:
– Composed of Glucose units
– Source: Main energy storage in animals; stored in the liver and muscles.
iii Cellulose:
– Composed of Glucose units
– Function: Structural component in plant cell walls; dietary fibre in human diet.
Sugar as a reducing agent
Sugar can act as a reducing agent in chemical reactions due to its ability to donate electrons. In processes like Maillard browning or caramelization, sugars reduce other compounds, leading to colour and flavour changes in food.
In Maillard browning, reducing sugars like glucose and fructose react with amino acids, particularly the amino group of proteins, leading to the browning of food and the development of complex flavours.
Reducing sugar + Amino acid Maillard reaction products
This reaction is responsible for the browning of bread crusts, the colour of roasted coffee beans, and the rich flavour in many cooked foods.
Sugar as a non-reducing agent
While most monosaccharides are reducing sugars, certain disaccharides like sucrose are non-reducing sugars. Sucrose consists of glucose and fructose linked by a glycosidic bond between their anomeric carbon atoms.
The glycosidic bond prevents sucrose from easily donating electrons, making it a non-reducing sugar. When sucrose undergoes hydrolysis, breaking this bond with the help of enzymes or acid, it forms glucose and fructose, both of which are reducing sugars:
Sucrose + Water Enzymes or Acid}Glucose + Fructose
Once broken down into glucose and fructose, these components can participate in reducing reactions.
EVALUATION:
- Identify the sources of sugar
- Classify sugar under the following heading
- Monosaccharides
- Disaccharides
- What is the function of sugar as a reducing agent?
CLASSWORK: As in evaluation
CONCLUSION: The teacher commends the students positively.