Water SS2 Chemistry Lesson Note
Download Lesson NoteTopic: Water

Water is regarded as the universal solvent. It is a good solvent for many substances.
SOURCES OF WATER
The following are the sources of water:
- Natural water: Rainwater, Well water, Spring water and seawater
- Treated water: Distilled water, Pipe–borne water and chlorinated water.
TYPES OF WATER
Water is of two types namely: soft water and hard water. Soft water forms lather with soap easily while hard water does not form lather readily with soap since it contains some dissolved salt in it.
STRUCTURE OF WATER
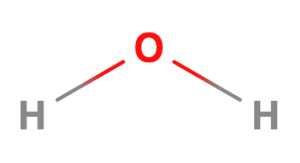
In a molecule of water, H2O, the central atom is Oxygen. Oxygen has the following electronic configuration: 1s² 2s² 2p⁴.
The valence shell of oxygen has two lone pairs of electrons (2s²2p²) and two unpaired electrons (2py12pz1). Each unpaired electron forms a covalent bond with an electron from a hydrogen atom. The water molecule has two lone pairs and two bond pairs of electrons in the valence shell of its central atom, thereby satisfying the octet rule for stability.
Ideally, the four electron pairs should be directed towards the corners of a tetrahedron. However, when lone pairs of electrons are located near another lone pair, the repulsion between them is so great that they squeeze the other two bond pairs of electrons closer together. As a result, the bond angle in water is compressed to approximately 105°, such that the structure of the water molecule is V-shaped or angular.
LABORATORY PREPARATION OF WATER
To prepare water in the laboratory, dry hydrogen gas is ignited in the air. It burns with a faint blue flame to give steam, which will condense on contact with any cold surface to form water.
PHYSICAL PROPERTIES OF WATER
- Water boils at 100°C and freezes at 0°C
- It has a maximum density of 1 g cm-3 at 4°C
- It is neutral to litmus.
CHEMICAL PROPERTIES
- Water reacts with electropositive metals to form alkali and liberate hydrogen gas. E.g
Na(s) + H2O(aq) > NaOH(aq) + H2(g)
Mg & Zn react with steam
Cu, Au, Ag, and Hg do not react with water to form an alkaline solution
- Non-metal-like chlorine reacts with water to form an acid solution.
H20(aq) + Cl2(g) > HCl(aq) + HOCl(aq)
TEST FOR WATER
When a few drops of water are added to:
- White anhydrous copper (II) tetraoxosulphate (VI), it turns blue.
- Blue cobalt (II) chloride, it turns pink.
NOTE: These two tests are not specific for water. They only indicate the presence of water. Any aqueous solution or substance containing water will give a positive test for water.
HARDNESS OF WATER
Hard water is water that does not form lather readily with soap.
Water acquires hardness when insoluble salts of CaSO4, MgSO4 and Ca(HCO3)2 dissolve in it from the soil which it flows through.
Types Of Hardness Of Water
- Temporary hard water
- Permanent hard water
I. Temporary Hardness: This is caused by the presence of Ca2+ and Mg2+ in the form of hydrogen trioxocarbonate IV i.e. Ca(HCO3)2
Removal Of Temporary Hardness
- Physical method: By boiling
Ca(HCO3)2(aq)heat > CaCO3(s) + H2O(l) + CO2(g)
- Chemical method: By using slaked lime (calcium hydroxide solution)
Ca(HCO3)2(aq) + Ca(OH)2(aq) > 2CaCO3(s)+ 2H2O(l)
Effects Of Temporary Hardness:
It causes:
- Furring of kettles and boilers.
- Stalagmite and stalactites in caves.
- Permanent Hardness
Permanent hardness in water is caused by the presence of Calcium and Magnesium ions in the form of soluble tetraoxosulphate (VI) and chlorides (i.e. CaSO4, MgSO4, MgCl2, CaCl2)
Removal of permanent hardness: By chemical method only
- Addition of washing soda
Na2CO3(aq) + CaSO4(aq) > CaCO3(s) + Na2SO4(aq)
- Addition of caustic soda
2NaOH(aq) + CaSO4(aq) > Ca(OH)2(s) + Na2SO4(aq)
- Ion exchange resin
CaSO4(aq) + Sodium zeolite > Calcium zeolite + NaSO4(aq)
(insoluble)
Advantages Of Hard Water
- It tastes better than soft water.
- Calcium salts in it help to build strong teeth and bones.
- It provides CaCO3, which crabs and snails use to build their shells.
- It does not dissolve lead, hence it can be supplied in lead pipes.
Disadvantages Of Hard Water
- It causes furring of kettles and boilers.
- It wastes soap.
- It cannot be used in dying and tanning.
TREATMENT OF WATER FOR MUNICIPAL SUPPLY
The following are the processes of treating river water for town supply:
- Coagulation: Chemicals like potash alum, KAl(SO4)2, or sodium aluminate III, NaAlO2 is added to water in a large settling tank.
- Sedimentation: The coagulated solid particles or flocs are allowed to settle in the settling tank to form sediments at the bottom of the tank.
- Filtration: The water above the sediment still contains some suspended particles. The water is passed through a filter bed to remove the remaining fine dirt particles.
- Chlorination (Disinfection): Chemicals like chlorine are then added to the water to kill germs. Iodine and fluorine are also added as food supplements to prevent goitre and tooth decay respectively. The treated water is then stored in a reservoir and distributed to the town.
ASSIGNMENT
Write the correct option ONLY
- Treated town water undergoes the following steps except A. coagulation B. precipitation C. sedimentation D. chlorination
- Water is temporarily hard because it contains A. CaSO4 B. MgSO4 C. chlorine D. Ca(HCO3)2
- Temporary hardness of water is removed by the use of one of the following A. boiling B. use of use of Ca(OH)2 C. use of Na2CO3 D. use of alum
- A substance that turns white anhydrous CuSO4 blue is A. water B. liquid ammonia C. hydrochloric acid D. molten sulphur
- Distilled water is different from deionized water because
A. distilled water is a product of condensed steam while deionized water is filtered laboratory water
B. distilled water is always pure and sold in packs while deionized is not packaged for consumption
C. distilled water is condensed steam but deionized water is produced using ion-exchange resins which absorb undesired ions.
D. distilled water is man-made while deionized water is both natural and artificial