Volumetric/Quantitative Analysis SS2 Chemistry Lesson Note
Download Lesson NoteTopic: Volumetric/Quantitative Analysis
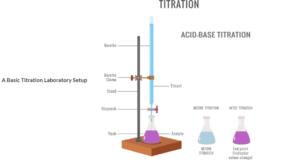
TITRATION
There are two types of quantitative analysis namely: volumetric and gravimetric analysis. Volumetric analysis is based on volume measurement while gravimetric analysis involves direct mass measurement.
Volumetric analysis is carried out using Titration. In a titration, a standard solution (one of known concentration must be used to react with a solution of unknown concentration)
PREPARATION OF A STANDARD SOLUTION
A standard solution is a solution of which the concentration is known. A standard solution is prepared by weighing a pure solute, for instance, and dissolving it in a suitable solvent, usually water, and making up the solution to a definite volume in a volumetric flask.
For instance, a solution known to contain exactly 10.6g of anhydrous sodium trioxocarbonate (IV), Na2CO3, in 1 dm3 of solution is a standard solution.
Preparation of 0.1mol/dm3NaOH
- 40g NaOH dissolved in 1 dm3 of the water gives 1.0mol/dm3 solution
- XgNaOH will be dissolved in 1 dm3 of water to give 0.1mol/dm3
Xg = 40g x 0.1mol/dm3
1.0mol/dm3
= 4g
Therefore, 4g of sodium hydroxide pellet is measured, dissolved in water and made up to 1dm3 mark to obtain 0.1mol/dm3NaOH
Preparation of 0.1mol/dm3HCl
To prepare 0.1mol/dm3HCl, the dilution formula is used to determine the volume of the stock acid that will be measured and dissolved in water to obtain the desired concentration.
The dilution formula is C1V1 = C2V2
Where C1 = concentration of stock acid = 11.6mol/dm3 (for HCl)
V1 = volume of stock acid
C2 = desired concentration of acid = 0.1mol/dm3
V2 = volume of water = 1000cm3 (1dm3)
V1 = C2V2
= 0.1 x 1000 = 8.6cm3
C1 11.6
Thus, 8.6cm3 of the stock acid is measured using a measuring cylinder and added to water, then made up to 1dm3 to obtain 0.1 mol/dm3HCl.
INDICATORS FOR ACID/BASE TITRATION
Acid-base indicators are dyes that change colour according to the pH of the medium. The table below shows some titration and their suitable indicator:
| SN | ACID/BASE | INDICATORS |
| 1 | Strong acid and strong base | Methyl orange or Phenolphthalein |
| 2 | Strong acid and weak base | Methyl orange |
| 3 | Weak acid and strong base | Phenolphthalein |
| 4 | Weak acid and weak base | No suitable indicator |
CONCENTRATION
The concentration of a solution is the amount of solute in a given volume of the solution. It can be expressed as mol/dm3 or g/dm3.
- Molar Concentration
The molar concentration of a compound contains one mole or the molar mass of the compound in 1dm3 of the solution. The unit of molar concentration is mol/dm3
- Mass Concentration
The mass concentration of a compound is the mass of the compound contained in 1 dm3 of solution. The unit is g/dm3
Relationship Between Molar Concentration And Mass Concentration
Concentration = number of moles = n/V………………(i)
volume
Number of moles, n = C x V ……………………..(ii)
But, number of moles, n= m/M
Where M = molar mass and m = mass
Substituting n=m/M into …….(ii)
We have m/M = C x V
That is, m/V = C x M
But m/V = mass concentration
Therefore, mass concentration = molar concentration x molar mass = C x M
TITRATION REPORT
| Burette Reading in (cm³) | Rough | 1st Titration | 2nd Titration | 3rd Titration |
| Final Reading | 23.40 | 23.20 | 28.20 | 34.10 |
| Initial Reading | 0.00 | 10.00 | 05.00 | 11.00 |
| Vol. of acid used (titre) | 23.40 | 23.20 | 23.20 | 23.10 |
The average volume of acid used
= 23.20 + 23.20 + 23.10
3
= 23.17cm3
ASSIGNMENT
Write the correct option ONLY
- The indicator used when titrating a weak acid against a strong base is A. methyl orange B. phenolphthalein C. methyl red D. any indicator
- The colour of phenolphthalein in acids is A.blue B. red C. colourless D. yellow
- Which of the following formulae is direct for amount n? A. n=C/p B. n=M/m C. n=C x V D. n=C x m
- The mass concentration of a substance can be expressed as A. mass/density B. molar concentration/molar mass C. mass/volume D. number of moles x volume
- At the end point there is A. a colour change B. no change of colour C. decrease in mass D. an increase in mass
- Define the following terms
(a) molar concentration
(b) Equivalent point
- 160cm3 of distilled water is added to 40cm3 of 0.500mol/dm3 H2SO4 solution. Determine the concentration of the diluted solution.