Unsaturated Hydrocarbons SS2 Chemistry Lesson Note
Download Lesson NoteTopic: Unsaturated Hydrocarbons
UNSATURATED HYDROCARBONS
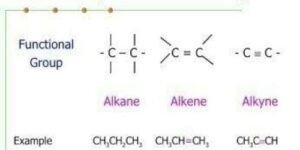
These are hydrocarbons in which carbon atoms join with each other by multiple bonds. The multiple bonds can be double bonds e.g. Alkenes or triple bonds e.g. Alkynes.
NOMENCLATURE
The process of naming in alkenes is obtained by substituting “and” in alkane with ‘ene’ e.g Ethane changes to Ethene, propane to propene
LABORATORY PREPARATION
- Ethene is prepared by heating ethanol with excess concentrated tetraoxosulphate(VI) acid at 170°C.
The acid acts as a dehydrating agent by removing water from the ethanol. Thus the process is called dehydration.
- The reaction occurs in two stages
- C2H5OH(aq) + H2SO4(aq) > C2H5HSO4(aq) + H2O(l)
- C2H5HSO4(aq) > C2H4(g) + H2SO4.
The overall reaction is represented by the equation:
C2H5OH(aq)H2SO4C2H4(g) + H2SO4(aq) > -H2O
PHYSICAL PROPERTIES
- Ethene is a colourless gas with a faint sweetish smell.
- It is sparingly soluble in water.
- It is slightly less dense than air.
- It has no action on litmus paper.
CHEMICAL PROPERTIES
- Combustion: Ethene undergoes combustion in air or the presence of oxygen and produces carbon (IV) oxide and steam.
C2H4(g) + 3O2(g) > 2CO2(g) + 2H2O(l)
- Addition Reaction: This is a reaction in which two molecules combine to form one molecule.
- Reaction with hydrogen (Hydrogenation)
- Reaction with halogens (Halogenation)
- Reaction with hydrogen halides(Halohydrogenation)
- Reaction with acidified /Alkaline KMnO4 (Hydroxylation): It decolourises acidified KMnO4, but turns alkaline KMnO4 to green and ethane -1,2- diol is formed.
- Reaction with Hydrogen peroxide in the presence of osmium trioxide to form ethane -1,2- diol.
- Reaction with concentrated H2SO4 produces a fuming liquid (ethyl hydrogen sulphate)
C2H4 + H2SO4 > C2H5HSO4
When ethyl hydrogen sulphate is hydrolyzed, tetraoxosulphate (VI) acid and ethanol are produced.
C2H5SO4 > C2H5OH + H2SO4
- Ethene gas decolourises bromine water to produce bromoethanol.
- Polymerization of ethane to produce polythene.
- Ethene can also undergo an additional reaction with oxygen in the presence of a silver catalyst at about250oC to form epoxy ethane.
USES OF ETHENE:
Ethene is used:
- In the manufacture of plastics.
- In making synthetic rubber.
- To hasten the ripening of fruits.
- In the production of other organic compounds e.g. halo-alkane, ethane and ethanol.
ALKYNES
Nomenclature
- Alkynes are the homologous series of unsaturated hydrocarbons with a general molecular formula CnH2n-2.
- Alkynes show a higher degree of unsaturation than alkenes, hence, they are chemically more reactive than the corresponding alkenes or alkanes.
- They are named by replacing the ‘and’ of alkanes with ‘one’.
ETHYNE
Ethyne is the first member of the alkynes series. It has a molecular formula, C2H2, and a structural formula of HC = CH.
LABORATORY PREPARATION
Ethyne is usually prepared in the laboratory by the action of cold water on calcium carbide. The reaction is carried out on a heap of sand to prevent the flask from cracking as a result of the large quantity of heat evolved.
PHYSICAL PROPERTIES
- Ethyne is a colourless gas with a characteristic sweet smell when pure.
- It is only sparingly soluble in water
- It is slightly less dense than air.
- It is unstable and may explode on compression to liquid.
CHEMICAL PROPERTIES
- Combustion: It undergoes a combustion reaction in air to form water and carbon(IV) oxide
2C2H2(g) + 5O2(g) > 2H2O(l) + 4CO2(g)
NB: In limited air, it burns with a very smoky and luminous flame because of its high carbon content. But in plenty of air and appropriate proportion, it burns with a non-luminous very hot flame of about 3000oC.
- Addition Reaction: Ethyne undergoes an additional reaction to produce an unsaturated product with double bonds and then a saturated compound with a single bond.
- Reaction with hydrogen in the presence of nickel as a catalyst.
- Reaction with halogens:
- Reaction with hydrogen halide: Hydrogen halide reacts with ethyne to produce halo-alkene and further halogenation produces halo-alkane.
- Reaction with water: When ethyne is passed through dilute tetraoxosulphate (vi) acid in the presence of mercury (II) tetraoxosulphate (VI) as a catalyst, the addition of water takes place to form ethanal.
- Reaction with acidified KMnO4: If ethyne is added to acidified KMnO4, it decolourises it. But with alkaline KMnO4, the solution turns to green.
- Polymerization: In the presence of complex organic –nickel as catalyst ethyne polymerizes to produce benzene.
3 C2H2 > C6H6
- Substitution Reaction
- Ethyne reacts with an ammoniacal solution of copper (1) chloride to form a reddish-brown solution of copper (I) dicarbide
C2H2 + 2CuCl > Cu2C2 + 2HCl
- With ammoniacal silver trioxonitrate (v), ethyne forms white silver dicarbide
C2H2 + 2AgNO3Ag2C2 + 2HNO3
These reactions to form dicarbide are used to distinguish ethyne from ethene.
USES OF ETHYNE:
Ethyne is:
- Mixed with oxygen to produce oxy-ethyne flame for cutting and welding of metals.
- Used in the manufacture of PVC plastics.
- Used in a miner’s lamp as fuel.
- Used in making synthetic fibre.
TEST FOR UNSATURATION
Unsaturated compound decolorizes bromine water.
ASSIGNMENT
- How would you obtain ethanal from ethyne? Give the equation for the reaction.
- Describe how to prepare ethyne in the laboratory.
- What is resonance? Give the resonance structure of benzene.
- Explain why hydrogen fluoride exists as a liquid whereas hydrogen chloride is a gas at room temperature.
- Explain why HCl in water conducts electricity but HCl in methyl benzene does not conduct electricity.