Alkanols SS2 Chemistry Lesson Note
Download Lesson NoteTopic: Alkanols
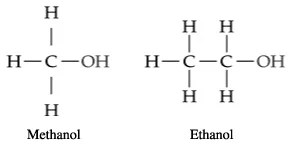
The functional group in alkanols is the hydroxyl (-OH) group.
NOMENCLATURE
The names of alkanols are obtained by substituting “e” in alkanes with “ol”.
Example:
Methanol – CH3OH, Ethanol – CH3CH2OH
CLASSIFICATION
The alkanols are classified based on the number of alkyl groups directly linked to the carbon atom carrying the hydroxyl group.
- Primary Alkanols: It has only one alkyl group attached to the carbon atom that carries the hydroxyl group.
- Secondary Alkanols: They have two alkyl groups directly linked with the carbon atom carrying the hydroxyl group.
- Tertiary Alkanols: The alkanols here have three alkyl groups attached to the carbon atom holding the hydroxyl group
TYPES OF ALKANOLS
The type of alkanols is determined by the number of the hydroxyl group –OH, present in the molecule.
- Monohydric Alkanols: This type has only one hydroxyl(–OH), present in its molecule.
Example: C2H5OH, C3H7OH.
- Dihydric Alkanols: This type has two hydroxyl groups per molecule.
- Polyhydric Alkanols: This type has three or more hydroxyl groups per molecule.
ETHANOL Laboratory Preparation
- Hydrolyzing ethyl esters with hot alkali.
- Reducing ethanol with nascent hydrogen.
COMMERCIAL PREPARATION
- From Ethene: Ethene is obtained by the cracking of petroleum. It is then absorbed in 95% H2SO4 at 800C and 30 atm to form ethyl hydrogen tetraoxosulphate (VI)
C2H4 + H2SO4 > C2H5HSO4
The ethyl hydrogen tetraoxosulphate (VI) is hydrolysed by boiling water to produce ethanol.
C2H5HSO4+ H2O > C2H5OH + H2SO4.
The ethanol is distilled off leaving the acid behind which can be used again.
- Preparation by Fermentation: Ethanol is prepared industrially from raw materials containing starch or sugar by the process of fermentation. Fermentation is an enzymatic process which involves the decomposition of large organic molecules into simple molecules by micro-organisms. The common microorganism used is YEAST
PRODUCTION OF ETHANOL FROM STARCHY FOOD
Ethanol can be prepared from starchy food like rice, potatoes, maize etc. The following steps are involved;
- Crush and pressure cook the starchy materials.
- Extract the starch granules by mixing them with water.
iii. Allow the starch granules to settle and decant.
- Treat the starch granules with malt (partially germinated barley which contains the enzyme, DIASTASE) at 500C for one hour.
- The starch is then converted to MALTOSE.
2(C6H10O5)n + nH2O > nC12H22O11
- Then yeast is added at room temperature for some time (at least one day). Yeast contains two enzymes, namely MALTASE and ZYMASE. Maltase converts maltose to two glucose units, while Zymase converts the glucose to ethanol and carbon (IV) oxide.
C12H22O11 + H2O > maltase 2C6H12O6
C6 H12 O6 Zymase > 2C2H3OH + CO2
Ethanol
PHYSICAL PROPERTIES
- Ethanol is a colourless volatile liquid.
- It is soluble in water.
- It has a boiling point of 780C.
- It has no action on litmus paper.
CHEMICAL PROPERTIES
- Combustion: The lower members of alkanols burn with clean flames in plenty of air
2CH3OH + 3O2 > 2CO2 + H2O
- Oxidation: The products of oxidation depend on the structure of the alkanol.
- Primary alkanols are oxidized to alkanol first and then to alkanoic acid in the presence of oxidizing agent e.g KMnO4
CH3CH2OH O > CH3CHO > CH3COOH
- Secondary alkanols oxidize to alkanone.
iii. Tertiary alkanols are not oxidized because there is no carbon-hydrogen bond to be broken for the oxidation to take place.
Note: The colour change of oxidizing agent if acidified is purple KMnO4 changes to colourless and the range K2Cr2O7 turns green.
- Esterification: This is the reversible reaction between alkanol and alkanoic to produce a sweet-smelling compound known as ester. The reaction is catalyzed by concentrated H2SO4.
Example
CH3CH2OH + CH3COOH > H + CH3COOCH2CH3 + H2O
- Dehydration: Alkanols are dehydrated to alkenes in the presence of concentrated H2SO4.
CH3CH2OH + H2SO4 > CH3CH2HSO4 + H2O > CH3CH2HSO4170oCC2H4 + H2SO4
- Reaction with sodium and potassium: Sodium and potassium react vigorously with alkanols to liberate hydrogen gas and form corresponding organic salt of sodium and potassium.
2C2H3OH + Na > 2C2H3ONa + H2
- Reactions with the chlorides of phosphorus: Ethanol reacts vigorously with PCl5 in the cold to produce fumes of HCl and chloroethane vapour.
C2H5OH + PCl5C2H5Cl + POCl3 + HCl
PCl3 gives a similar reaction, but less vigorous.
C2H5OH + PCl3 3C2H5Cl + H3PO3
USES OF ETHANOL
- It is used as an organic solvent.
- It is the main constituent of the methylated spirit used to clean wounds and dissolve paint.
- It is used as a petrol additive for use as fuel in vehicles.
- It is used to manufacture other chemicals such as ethanol and ethanoic acid.
- It is used as an ingredient in making alcoholic drinks e.g. beers, wines and spirits.
- It is used as an antifreeze in automobile radiators because of its low freezing point (-117°C).