Gas Laws SS2 Physics Lesson Note
Download Lesson NoteTopic: Gas Laws
In an attempt to study the behaviour of gases concerning volume, temperature and pressure, the following conditions are investigated:
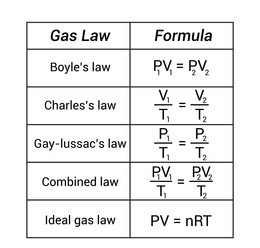
- variation of volume with pressure at constant temperature, Boyle’s law (PV = constant)
- variation of volume with temperature at constant pressure,
- Charles law ( V÷T = constant)
- variation of pressure with temperature at constant volume, pressure law (P ÷ T = K)
BOYLE’S LAW
Boyle’s law states that the pressure of a fixed mass of gas varies inversely to the volume at a constant temperature.
P ~ 1/V …….1
P = K/V ……2
PV = K …….3
P1V1 = P2V2 ..…..4
CHARLES’ LAW
Charles’s law states that for a fixed mass of gas at constant pressure, the volume is proportional to its absolute temperature
V ~ T ….1
V/T = constant …..2
V1/T1 = V2/T2 ….3
PRESSURE LAW
Pressure law states that the pressure of a fixed mass of gas at constant volume is proportional to its absolute temperature.
P ~ T ….1
P/T = constant ….2
P1/T1 = P2/T2 ….3
ABSOLUTE ZERO OF TEMPERATURE
When the graphs of volume–temperature or pressure-temperature are extrapolated backwards they cut the temperature axis at -273°C. This temperature is called absolute zero, the temperature at which the volume of the gas theoretically becomes zero as it is being cooled.
At his temperature, molecules of gas stop moving completely. This temperature is a mere assumption, as gases are known to liquefy more often than not before such a temperature is reached.
GENERAL GAS LAW
The general gas law is a combination of Boyle’s, Charles and Pressure’s Law.
It follows that
From Boyle’s law: PV = K
From Charles’ law: V/T = K
From Gay-Lussac’s or Pressure law:
P/T = K
In combination, we have:
P1V1 = P2V2
T1 T2
This equation is known as general gas law or relation. This can be written in the form:
PV = nRT Where: n=number of moles of gas
R=the universal molar gas constant = 8.31JK-1
ASSIGNMENT
A vessel is filled with a gas at a temperature of 50°C and a pressure of 76cmHg. Calculate the final pressure if the volume of the gas is doubled while it is heated to 90°C