Sulphur SS2 Chemistry Lesson Note
Download Lesson NoteTopic: Sulphur
GENERAL PROPERTIES OF THE SULPHUR GROUP (GROUP VI ELEMENTS)
- The group VI elements include Oxygen, Sulphur, Selenium, Tellurium and Polonium.
- Metallic property increases down the group. Oxygen and sulphur are non-metal; selenium and tellurium are metalloid; while polonium is a metal.
- All the elements are solid except oxygen which is a gas at room temperature.
- Oxygen and sulphur exhibit allotropy.
- They have six electrons in their outermost shell. Hence their oxidation number is -2; though sulphur can exhibit -4 and -6 states in some compounds.
- Electronegativity decreases down the group. Thus, oxygen is a good oxidizing agent.
ELECTRONIC STRUCTURE OF SULPHUR GROUP
Members of the sulphur family include Oxygen, Sulphur, Selenium, Tellurium and Polonium. Their electronic configurations are shown below:
Oxygen = 8: 1s² 2s² 2p⁴
Sulphur = 16: 1s² 2s² 2p⁶ 3s² 3p⁴
Selenium= 34: 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁴
Tellurium = 52: 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 5s² 5p⁴
Polonium = 84: 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 5s² 5p⁶ 5d¹⁰ 5f¹⁴ 6s² 6p⁴
SULPHUR
Sulphur is an element. It occurs freely as deposits and in a combined state as sulphide and as tetraoxosulphate (IV).
EXTRACTION OF SULPHUR
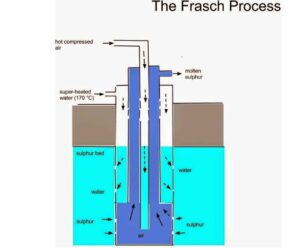
Sulphur is extracted from underground by the Frasch process. A three-concentric sulphur pump is driven down a hole dug into the sulphur bed. The solid sulphur is melted at 115oC by superheated water at 170oC and 10 atms. The molten sulphur is forced out by hot compressed air at 15atm. The molten sulphur is then continuously pumped out and allowed to solidify in a large tank. The sulphur obtained is 99.5% pure.
ALLOTROPES OF SULPHUR
The two main crystalline allotropes of sulphur are:
- Rhombic sulphur: A bright yellow octahedral crystalline solid. Each crystal is made up of S8 molecules. Rhombic sulphur is stable below 96oC.
- Monoclinic Sulphur: This is amber-coloured solid sulphur consisting of needle-shaped S8 crystal. Stable above 96oC. It easily reverts to Rhombic below 96oC. The transition temperature between Rhombic and Monoclinic is 96oC.
COMPARISON OF THE PHYSICAL PROPERTIES OF RHOMBIC AND MONOCLINIC SULPHUR
- Rhombic Sulphur melts at 113°C;while monoclinic sulphur melts at 119°C.
- Rhombic sulphur has bright yellow colour; while monoclinic sulphur has amber colour.
- Rhombic sulphur has an octahedral shape; while monoclinic sulphur is needle-like in shape.
- Rhombic sulphur is translucent; while monoclinic is transparent.
- Sulphur also exists as a non – crystalline solid. These are:
- Amorphous sulphur
- Plastic sulphur
PHYSICAL PROPERTIES
- Sulphur is a yellow solid.
- It is insoluble in water but soluble in toluene and carbon (IV) sulphide.
- It is a poor – conductor of heat and electricity.
- It melts at 119°C and boils at 444°C.
CHEMICAL PROPERTIES
- It reacts directly with metals to form sulphide (S2-)
Fe(s) + S(s) → FeS(s)
- It reacts with excess oxygen to form sulphur (IV) oxide
O2(g) + S(s) → SO2(g)
- It reacts with hydrogen to form hydrogen sulphide;
H2(g) + S(s) → H2S(g)
- It reacts with coke (carbon) to form carbon (IV) sulphide.
C(s) + 2S(s) → CS2(l)
USES
- Used in manufacturing tetraoxosulphate (IV) acid
- Used in vulcanization of rubber
- Used as germicides
- Used in manufacturing bleaching agent
COMPOUNDS OF SULPHUR
- Hydrogen Sulphide, H2S
Hydrogen sulphide is found in volcanic gases, sulphur springs, coal gas and natural gas.
Laboratory Preparation
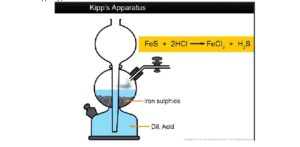
i. Hydrogen sulphide is prepared in the laboratory by the action of dilute acids on metallic sulphide like Iron (II) sulphide
2HCl(aq) + FeS(s) → FeCl2(aq) + H2S(g)
ii. The apparatus used for the regular supply of hydrogen sulphide in the laboratory is Kipp’s apparatus.
Physical Properties
- Hydrogen sulphide is a colourless gas with a smell like that of rotten egg.
- It is very poisonous.
- It is about 1.18 times denser than air.
- It is moderately soluble in water to form a very weak acidic solution.
It burns with a pale blue flame.
Chemical Properties
i. As an acid, it reacts with alkali to form a normal salt and water
2NaOH(aq) + H2S(g) → Na2S(aq) + 2H2O(l)
ii. It reacts with excess oxygen to form sulphur (VI) oxide but forms a deposit of sulphur with limited oxygen
2H2S(g) + 3O2(g) → 2H2O(l) + 2SO2(g)
2H2S(g) + O2(g) → 2H2(l) + 2S(s)
iii. As a reducing agent, it reacts with many oxidizing agents such as acidified KMnO4, acidified K2Cr2O7, chlorine gas, FeCl2, SO2, H2SO4 and HNO3
Test For Hydrogen Sulphide
A piece of filter paper is moistened with lead (II) trioxonitrate (V) solution and dropped into a gas jar of the unknown gas. If the paper turns black, then the gas is H2S.
- Sulphur (Iv) Oxide, SO2
Laboratory Preparation
Sulphur (IV) oxide is prepared in the laboratory by heating sodium or potassium thiosulphate (IV) with tetraoxosulphate (IV) acid or hydrochloric acid.
Na2SO3(aq) + 2HCl(aq) → 2NaCl(aq) + H2O(l) + SO2(g)
Physical Properties
- Sulphur (IV) oxide is a colourless poisonous gas with a smell like that of burning matches.
- It is very soluble in water.
- It is about 2.5 times denser than air.
Chemical Properties
i. As an acid, it reacts with alkali to form normal salt of trioxosulphate and water only
2NaOH(aq) + SO2(g) → Na2SO3(aq) + H2O(l
ii. As a reducing agent, sulphur (IV) oxide reacts with many oxidizing agents such as acidified KMnO4; acidified K2Cr2O7; FeCl3, HNO3, and chlorine gas.
iii. It decolorizes acidified purple KMnO4 and turns acidified orange K2Cr2O7 to green.
iv It reacts as a bleaching agent decolourising dye by its bleaching action. The bleaching action is similar to that of chlorine in that there must be water. But, while chlorine bleaches by oxidation sulphur IV oxide bleaches by reduction.
v Sulphur (IV) oxide reacts as an oxidizing agent in the presence of a strong reducing agent such as hydrogen sulphide.
2H2S(g) + SO2(g) → 2H2O(l) + 3S(s)
C(s) + SO2(g) → CO2(g) + S(s)
Test For SO2
- If an unknown gas bleaches a coloured flower, SO2 can be suspected
- The unknown gas bubbled through a solution of either acidified potassium heptaoxodichromate (VI) or potassium tetraoxomanganate (VII). If the orange colour of the acidified K2Cr2O7 turns green or the purple colour of the acidified KMnO4 turns colourless, then the unknown gas is SO2
Uses
- It is used in the manufacture of tetraoxosulphate (VI) acid.
- It is used as a germicide and a fumigant, especially for destroying termites.
- It is used as a bleaching agent for straw, silt and wood.
- It is used as a preservative in some liquids e.g. orange juice.
- Liquid sulphur (IV) oxide is used as a refrigerant.
- Sulphur (Vi) Oxide, SO3
Sulphur (VI) oxide is prepared by reacting sulphur (IV) oxide and oxygen under special conditions which are:
i. Presence of platinized asbestos or vanadium (V) oxide as a catalyst
ii. Pressure of 1atm
iii. Temperature range of 400oC – 450°C.
2SO2(g) + O2(g) 2SO3(g)
Physical Properties Of So3
- It exists as white needle-like crystals at room temperature.
- It has a low boiling point and vaporizers on gentle heating.
- It dissolves readily in water to give tetraoxosulphate (VI) acid.
4. Trioxosulphate IV Acid, H2SO3
Trioxosulphate (IV) acid is a dibasic acid with a molecular formula H2SO3
Laboratory Preparation Of H2SO3
It is prepared by the action of dilute hydrochloric acid on heated sodium tetraoxosulphate (IV) to produce sulphur (IV) oxide, which is then dissolved in water.
Na2SO3(s) + 2HCl(aq) → 2NaCl(aq) + H2O(l) + S02(g)
H2O(l) + SO2(g) H2SO3(aq)
Sulphur (IV) Oxide is the acid anhydride of trioxosulphate (IV) acid.
Physical Properties Of H2SO3
- It turns blue litmus paper red.
- It is a colourless liquid.
- It mixes readily with water.
- It has an irritating and choking smell.
Chemical Properties Of H2SO3
i. It reacts with alkalis to form salt and water.
2NaOH(aq) + H2SO3(aq) → Na2SO3(aq) + 2H2O(l)
ii. It is oxidized in air to tetraoxosulphate (VI) acid
2H2SO3(aq) + O2(g) → 2H2SO4(aq)
iii. It reduces oxidizing agents such as potassium tetraoxomanganate (VII) and potassium heptaoxodichromate (VI).
iv. It bleaches dyes in the presence of water.
Test For SO3²-
Barium chloride solution is added to the solution of the unknown substance. A white
precipitate soluble in dilute hydrochloric acid confirms the presence of a trioxosulphate (IV)
ion.
Uses Of H2SO3
- It is used for bleaching straw and other fabrics.
- It is used as a germicide.
- Tetraoxosulphate Vi Acid, H2SO4
Tetraoxosulphate VI acid is one of the most important chemical compounds known. It is used in almost every manufacturing process; hence it is mostly prepared industrially.
Industrial Preparation Of H2SO4
Industrially, tetraoxosulphate VI acid is manufactured by the Contact process. The following steps are involved in the Contact process.
i. Burning sulphur in dry air to obtain sulphur (IV) oxide, SO2
S(s) + O2(g) → SO2(g)
ii. The sulphur (IV) oxide produced is mixed with excess air and passed through an electric chamber to remove impurities and dust which may poison the catalyst. The gaseous mixture is then passed through concentrated H2SO4 to dry it before passing it into the reaction chamber.
iii. The dried gaseous mixture is delivered to the contact tower (reaction chamber) where the sulphur (IV) oxide and oxygen combine in the presence of pellets of catalyst, vanadium (V) oxide, and V2O5 to yield sulphur (VI) oxide. The reaction takes place at atmospheric pressure and temperature of 450-500oC.
2SO2(g) + O2(g) 2SO3(g) + heat
iv. The sulphur (VI) oxide is cooled and then dissolved in concentrated H2SO4 to produce a thick liquid called Oleum.
H2SO4(aq) + SO3(g) → H2S2O7(aq)
v. The Oleum is then diluted with water appropriately to produce 98% tetraoxosulphate (VI) acid.
H2O(l) + H2S2O7(aq) → 2H2SO4(aq)
NOTE: Sulphur (VI) oxide is not dissolved directly in water to produce the acid because of the large amount of heat that is evolved in the process. The heat is capable of boiling the acid formed to produce a mist of droplets which can spread throughout the factory and cause acid burns.
Physical Properties
- It is a colourless, viscous liquid with a density of 1.84g cm-3
- It is corrosive and cause burns when in contact with the skin.
- It is highly soluble in water, evolving large amounts of heat.
Chemical Properties
i. As an acid, it reacts with metals which are above hydrogen in the electrochemical series to liberate hydrogen gas
Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g)
ii. It reacts with bases to form salts and water
MgO(s) + H2SO4(aq) → MgSO4(aq) + H2O(l)
iii. It reacts with alkali to form normal and acidic salt
H2SO4(aq) + NaOH(aq) → NaHSO4(aq) + H2O(l)
H2SO4(aq) + NaOH(aq) → Na2SO4(aq) + 2H2O(l)
iv. As an acid, it reacts with trioxocarbonate (IV) to liberate carbon (IV) oxide
H2SO4(aq) + CuCO3(aq) → CuSO4(aq) + H2O(l) + CO2(g)
v. As an oxidizing agent, concentrated H2SO4 oxidizes metals, non–metals and hydrogen sulphide in the following ways.
Zn(s) + 2H2SO4(aq) → ZnSO4(aq) + 2H2O(l) + SO2(g)
C(s) + 2H2SO4(aq) → 2H2O(l) + CO2(g) + 2SO2(g)
H2SO4(aq) + H2S(g) → S(s) + H2O(l) + SO2(g)
vi. Concentrated tetraoxosulphate (VI) acid is a dehydrating agent, removing water from compounds like sugar, ethanol, methanoic acid and ethanedioic acid
C12H22O11(s) → 12C(s) + 11H2O(l)
sugar charcoal
vii. Concentrated tetraoxosulphate (VI) displaces volatile acids from their salts
KCl(s) + H2SO4(aq) → KHSO4(aq) + HCl(g)
Test For SO42-
Barium chloride solution is added to the solution of the unknown substance. A white precipitate insoluble in excess dilute hydrochloric acid confirms the presence of a tetraoxosulphate (VI) ion.
Uses Of H2SO4
- It is used in the production of fertilizers e.g. ammonium tetraoxosulphate (VI).
- It is used in the purification of crude oil.
- It is used as an electrolyte in lead-acid accumulators.
- It is used as a drying agent for many gases except NH3 and H2S gas.
- It is used to clean metals before electroplating.
Uses Of Tetraoxosulphate (Vi) Salts
- Ammonium tetraoxosulphate (VI) used as fertilizer
- Sodium tetraoxosulphate (VI) is used in paper manufacture and as a purgative
- Calcium tetraoxosulphate (VI) is mined as gypsum and when heated forms plaster of Paris used to set broken bones.
- Aluminum tetraoxosulphate (VI) is used to coagulate precipitate in the purification of water
- Iron II tetraoxosulphate (VI) is used to treat anaemia.