Evaporation SS2 Physics Lesson Note
Download Lesson NoteTopic: Evaporation
EVAPORATION
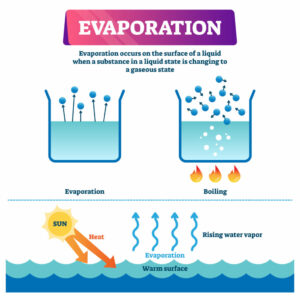
Evaporation is defined as the process by which liquid molecules break away from the surface of the liquid to remain as vapour. It can also be simply defined as the process by which liquid changes to gas or vapour.
BOILING POINT
As heat is added to a liquid, its temperature increases steadily until at a certain temperature when rapid evaporation is seen to occur in every part of the liquid with the bubbles of vapour escaping to the surface. This process is known as boiling.
As a matter of distinction, evaporation occurs only at the surface of the liquid while boiling occurs throughout the entire mass of the liquid. Also, evaporation takes place at all temperatures but boiling occurs at a particular temperature called boiling point.
EFFECTS OF PRESSURE ON BOILING
An increase in pressure at the surface of a liquid raises the boiling point of the liquid and conversely, a decrease in pressure lowers the boiling point of the liquid.
EFFECTS OF IMPURITIES ON BOILING
The presence of impurities or dissolved substances in a liquid raises the boiling point of the liquid but lowers the freezing point
MELTING POINTS
The melting point is the temperature at which a solid substance has its bond broken such that it now flows as a liquid. It is also defined as the constant temperature at which a substance changes state from solid to liquid. On the reverse, the constant temperature at which a substance changes state from liquid to solid is called the freezing point
APPLICATION IN PRESSURE COOKER
The fact that increased pressure raises the boiling point is put into a useful application in the pressure cooker. The increased pressure of the trapped gas above the liquid raises the boiling of the liquid inside the cooker. This provides a high cooking temperature needed to conserve fuel and save time.
APPLICATION IN REFRIGERATORS
Refrigerators make use of the cooling effect of evaporation. The volatile liquid such as liquid ammonia or Freon evaporates inside the copper coil surrounding the freezing compartment, supported by an electric pump which reduces the pressure. As the volatile liquid evaporates in those coils, it absorbs heat from the surrounding air, consequently cooling the inside of the refrigerator and its contents.
The vapour produced is pumped off into the condenser, where it is compressed by the pump and condenses back to liquid. The latent heat given out during this condensation is quickly dissipated by an arrangement of cooling fins at the back of the refrigerator.
Heat is eliminated by convection and radiation to the surroundings and by conduction into fins. The liquid is again passed into the evaporator coil and thus the level of cooling is regulated by a thermostat connected to the switch.