Solubility SS2 Chemistry Lesson Note
Download Lesson NoteTopic: Solubility
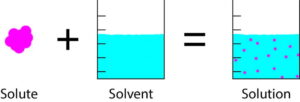
A solution is a uniform or homogenous mixture of two or more substances.
Solution = Solvent + Solute
A solute is a dissolved substance which may be a solid, liquid or gas.
A solvent is a substance (usually liquid) which dissolves a solute.
TYPES OF SOLUTIONS
- Aqueous Solution: This is formed when a solute is dissolved in water.
- Chemical Solution: This is the apparent solution of a solute in a solvent accompanied by a chemical change. For example, magnesium appears to dissolve in dilute hydrochloric acid, what happens is that the magnesium attacks the acid to form magnesium chloride, which dissolves in water present.
TRUE SOLUTION AND COLLOIDAL SOLUTION
A true solution is formed when solute particles dissolve such that they can get in between the solvent particles. An example of a true solution is an aqueous solution of sodium chloride and copper (II) tetraoxosulphate (VI).
A False or Colloidal solution is one in which the individual particles are larger than the particles of a true solution, but not large enough to be seen by the naked eye. Examples of colloids are starch and albumen.
Types Of Colloids
- Sols and Gels: These are colloids where solid particles are dispersed in a liquid medium. Example: starch, glue, jelly, etc
- Aerosols: In aerosols, liquid particles are dispersed in a gas. Fog, smoke, and spray of insecticide are examples of aerosol.
- Emulsion: For emulsions, a liquid is dispersed in another liquid. Examples of emulsions are milk and hair cream; the cleaning action of detergents is due to their ability to form emulsions.
SOLUBILITY
The solubility of a solute (substance) in a solvent at a particular temperature is the maximum amount of solute in moles or grams that will dissolve in 1 dm3 of the solvent at that temperature.
The concentration in mol dm-3 of a saturated solution is termed the solubility of the substance i.e. Solubility
(moldm³) = Concentration in gdm³
Molar mass
Solubility in mol/dm3 can also be expressed as
= mass x 1000
Molar mass volume
Solubility in g/dm3
= mass x 1000
volume 1
The solubility of a solid solute in a solvent increases with the rise in temperature while the solubility of gases decreases with the temperature rise.
DEFINITION OF TERMS
- Saturated Solution: A saturated solution at a particular temperature is one which contains as much solute as it can dissolve at that temperature in the presence of undissolved solute particles.
- Unsaturated Solution: This is a solution which contains less of the solute than it can dissolve at a particular temperature.
- Supersaturated Solution: This is a solution which contains more of the solute than it can dissolve at a particular temperature.
DETERMINATION OF SOLUBILITY
Solute: KCl, Solvent: water
Method
- A saturated solution of KCl is prepared by dissolving the excess of the solid in water in a beaker.
- Allow the solution in the beaker to settle down to obtain a clear saturated solution.
- Decant a portion of the clear solution into another beaker and measure its temperature.
- Transfer the solution into a weighted evaporation dish and record the mass of the solution.
- Evaporate the solution to a complete dryness in a water bath
- Allow the resulting solid to cool and reweigh the basin with content
- Obtain the mass of the dissolved salt and calculate the mass of the salt that would dissolve in 1dm3 of water at that temperature.
CALCULATION
Mass of basin = xg
Mass of basin + solution = yg
Mass of basin + salt = zg
Mass of solution = (y-x)g
Mass of salt = (z-x)g
The mass of water used = (y-z)g
:. (y – z)g H2O dissolves (z – x)g salt
:. 100g H2O dissolves (z – x)/(y – z) x 100g salt
[Density of water = 1gcm3]
:. No moles of salt
= 100(z – x)
(y-z) x M.M
:. Moles of salt dissolves in 1 dm3 water = 100(z-x) (y-z) x M.M
FACTORS THAT AFFECT SOLUBILITY
- Nature of solvent and solute
- Temperature
- Pressure (often neglected)
SOLUBILITY CURVES
These are the graphs of solubility against temperature. The graph provides a useful source of information.
Uses Of Solubility Curves
- It provides useful information about suitable solvents and temperatures for solvent extraction from natural sources.
- It provides useful information about temperature for fractional crystallization of a mixture of soluble salts.
- The curves enable pharmacists to determine the amount of solid drugs that must be dissolved in a given quantity of solvent to give a prescribed drug mixture.
CALCULATION ON SOLUBILITY
- If 12.2g of Pb(NO3)2 were dissolved in 21cm³ of distilled water at 20°C. Calculate the solubility of the solute in mould-
Solution:
Molar mass of Pb(NO3)2 = 331g
No of moles of Pb(NO3)2 = 12.2/331 = 0.037moles
If 21cm3 of water at 20°C dissolved 0.037mole salt
:. 1000cm3 of water at 20°C dissolves 0.037 x 1000/21
= 176 moles Pb(NO3) per dm3 H2O
- 1.0dm³ of an aqueous solution at 90°C contains 404g of KNO3 and 245g of KClO3.
- Determine which of the two salts will separate when the solution is cooled to 60°C
- mass of salt that will separate at 60°C
(Solubility of KNO3 in H2O at 60°C = 5.14mol dm-3, solubility of KClO3 in H2O at 60°C = 1.61mol dm-3)
Solution:
No of moles of KNO3 = 404/101 = 4.0moles dm-3
No of moles of KClO3 = 245/122.5 = 2.0 mol dm-3
The solubility of KClO3 at 60oC (5.14 mol dm-3) is higher than the amount in solution (4.0 mol dm-3), then KNO3 will remain in solution while KClO3 will crystallize out at 60oC since the solubility at 60oC is lower than the amount in solution.
- Mass of salt that will separate at 60 = 2.0 – 1.61 = 0.39mole
Mass of salt = Number of moles x Molar mass
= 0.39 x 122.5 = 47.78g
- The solubility of KNO3 is exactly 1800g per 1000 g water at 83°C and 700g per 1000g water at 40°C. Calculate the mass of KNO3 that will crystallize out of the solution if 155g of the saturated solution at 83°C is cooled to 40°C.
Solution:
Saturated solution of KNO3 at 83oC = 1000 + 1800 = 2800g
Saturated solution of KNO3 at 40oC = 1000 + 700 = 1700g
Mass of solute deposited = 2800 – 1700 = 1100g
From 83°C to 40°C, 2800 of saturated solution deposited 1100g of solute
155g of saturated solution will deposit 1100 x 155/2800 = 60.80g of salt.
ASSIGNMENT
- Calculate the solubility of KCl in g/dm3 if 5g of the salt was dissolved in 50cm³ of water at 40°C
- If 50cm³ of a saturated solution of potassium chloride at 30°C yielded 18.62g of dry salt, calculate the solubility of the salt in mol/dm3 at 30°C
- Define solubility.
- A certain mass of a gas occupies 300cm³ at 35°C. At what temperature will it have its volume reduced by half, assuming its pressure remains constant?
- A certain mass of hydrogen gas collected over water at 10°c and 760 mmHg pressure has a volume of 37cm³. Calculate the volume when it is dry at s.t.p. (Saturated vapour pressure of water at 10°C = 9.2mmHg)