Saturated Hydrocarbons SS2 Chemistry Lesson Note
Download Lesson NoteTopic: Saturated Hydrocarbons
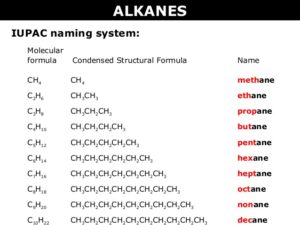
The alkanes are aliphatic hydrocarbons. Their general molecular formula is CnH2n+2. Hence
For n=1 CH4 Methane
n=2 C2H6 Ethane
n=3 C3H8 Propane
n=4 C4H10 Butane
n=5 C5H12 Pentane and so on.
There is no functional group in the alkane series.
THE IUPAC NOMENCLATURE FOR ALIPHATIC COMPOUNDS
In IUPAC nomenclature, every name of an organic compound consists of ROOT, SUFFIX and PREFIX names.
- Root name: Name of the parent aliphatic hydrocarbon of the longest carbon chain in a molecule.
- Suffix name: Name of the principal functional group on the longest carbon chain in a molecule.
- Prefix name: Name of the other substituents on the longest carbon chain which are not functional groups. For example, 1- chloroethane- 1- ol; has 1-chloro as a prefix, ethane as root and -1-ol as a suffix.
RULES FOR THE IUPAC NOMENCLATURE
- Select the longest continuous carbon chain as the parent hydrocarbon.
- Number the longest carbon chain starting from the end that gives the lowest possible number to the suffix (functional group).
- Indicate the other substituents by prefixes preceded by numbers to show their position on the carbon chain.
- If the same alkyl or other substituents group occurs more than once as a side chain, show how many are present by using the prefix di, tri, tetra etc and indicate by various numbers the position of each group on the longest carbon chain.
- If there are several different alkyl groups attached to the parent chain, name them in alphabetical order.
- If there are halogens together with alkyl groups attached to the parent chain, name the halogens first in alphabetical order and the alkyl group as explained earlier.
LABORATORY PREPARATION
- Methane is prepared in the laboratory by heating ethanoate salt with corresponding alkalis e.g. sodium ethanoate and soda-lime. Soda-lime is quick lime-slaked with a concentrated solution of sodium hydroxide. It is used in preference to caustic soda because it is not deliquescent and does not attack glass so readily.
PHYSICAL PROPERTIES
- Methane is a colourless and odourless gas.
- It is slightly soluble in water.
- It is less dense than air
- It has no action on litmus paper
CHEMICAL PROPERTIES
- Combustion: Methane burns in air or oxygen to produce steam, carbon(IV) oxide and a lot of heat
CH4(g) + 2O2(g) > 2H2O(l) + CO2(g).
The general equation for the combustion of alkanes is represented as
CxHy(g) + ( x + y/4)O2 y/ 2H2O(g) + xCO2(g).
- Substitution Reaction: Substitution reaction involves the direct displacement of an atom or group by another atom or group. Methane reacts with chlorine in the presence of ultraviolet light to yield a mixture of products.
CH4(g) + Cl2(g) > CH3Cl(g) + HCl(g)
USES OF METHANE
- Methane is used as fuel by itself or mixed with other gases.
- It is used for making hydrogen gas.
- It is used in making carbon black.
- Trichloromethane is used in surgical operations as an anaesthetic.
ISOMERISM
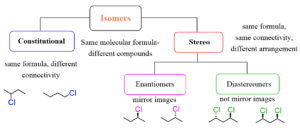
This is the existence of two or more organic compounds with the same molecular formula but different molecular structures.
Types Of Isomerism
- Structural Isomerism
- Stereoisomerism.
Structural isomerism occurs in organic compounds with the same molecular formula but different structural arrangements of the carbon atom.
Types Of Structural Isomerism
- Chain isomerism: This is the kind of isomerism which occurs due to the differences in the way by which the carbon atoms are arranged in the chain.
- Functional isomerism: This is the kind of isomerism which is due to the differences in functional groups.
iii. Positional isomerism: This is the kind of isomerism which occurs as a result of the difference in the way the functional group is positioned.
- Stereo Isomerism: This arises as a result of differences in spatial orientation of atoms or groups of atoms about a carbon-carbon double bond or ring structure or a carbon atom surrounded by four different groups.
Types Of Stereoisomerism
- Geometric isomerism: This is the existence of compounds with the same molecular formula but different spatial structural formula.
- Optical isomerism: They have different configurations and they rotate plane polarized light.







