Mass-Volume Relationship SS2 Chemistry Lesson Note
Download Lesson NoteTopic: Mass-Volume Relationship
MOLE AND MOLAR QUANTITIES
THE MOLE
A mole is several particles of a substance which may be atoms, ions, molecules or electrons. This number of particles is approximately 6.02 x 1023 in magnitude and is known as Avogadro’s number of particles.
The mole is defined as the amount of a substance which contains as many elementary units as there are atoms in 12g of Carbon-12.
RELATIVE ATOMIC MASS
The relative atomic mass of an element is the number of times the average mass of one atom of that element is heavier than one-twelfth the mass of one atom of Carbon-12. It indicates the mass of an atom of an element. For e.g, the relative atomic masses of hydrogen, oxygen, carbon, sodium and calcium are 1, 16, 12, 23, and 40 respectively.
The atomic mass of an element contains the same number of atoms which is 6.02 x 1023 atoms; 1 mole of hydrogen having an atomic mass of 2.0g contains 6.02 x 1023 atoms.
RELATIVE MOLECULAR MASS
The relative molecular mass of an element or compound is the number of times the average mass of one molecule of it is heavier than one-twelfth the mass of one atom of Carbon-12
It is the sum of the relative atomic masses of all atoms in one molecule of that substance. It is also called the formula mass. The formula mass refers not only to the relative mass of a molecule but also that of an ion or radical.
Calculation
Calculate the relative molecular mass of:
- Magnesium chloride
- Sodium hydroxide
iii. Calcium trioxocarbonate
[Mg=24, Cl=35.5, Na=23, O=16, H=1, Ca=40,C=12]
Solution:
- MgCl 2 = 24 + 35.5x 2 = 24 + 71 = 95 gmol-1
- NaOH = 23 + 16 + 1 = 40 gmol-1
iii. CaCO3 = 40 + 12 +16×3 = 100 gmol-1
MOLAR VOLUME OF GASES
The volume occupied by 1 mole of a gas at standard conditions of temperature and pressure (s.t.p) is 22.4 dm3. Thus 1 mole of oxygen gas of molar mass 32.0g mol-1 occupies a volume of 22.4dm3 at s.t.p and 1 mole of helium gas of molar mass 4.0gmol-1 occupies a volume of 22.4 dm3 at s.t.p.
Note: When the conditions of temperature and pressure are altered, the molar volume will also change. Also, standard temperature = 273 K and standard pressure = 760mmHg.
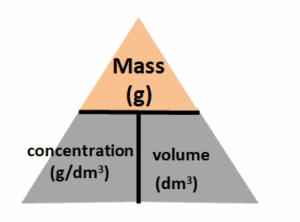
RELATIONSHIP BETWEEN QUANTITIES
Molar mass = mass (g)
Amount (moles) (n)
i.e. M = m gaol-1
n
Note: Amount = Number of moles
Molar volume of gas
= volume (cm³ or dm³)
Amount (mole) (n)
Amount = Reacting mass (g)
Molar mass (gmol-1)
Also, Amount of substance
= Number of particles
Avogadro’s constant
But, Avogadro’s constant = 6.02 x 1023
Combining the two expressions:
Reacting mass = Nos. of particles
Molar mass 6.02 x 1023
Calculations
- What is the mass of 2.7 moles of aluminium (Al=27)?
Solution:
Amount = Reacting mass
Molar mass
Reacting mass = Amount x Molar mass
= 2.7mole x 27 gmol-1 = 72.9g.
- What is the number of oxygen atoms in 32g of the gas? (O=16, NA = 6.02 x 1023)
Solution:
Reacting mass = Number of atoms
Molar mass 6.02 x 1023
Number of atoms
= Reacting mass x 6.02 x 1023
Molar mass
Molar mass of O2 = 16×2 =32 gmol-1
Number of atoms
= 32g x 6.02 x 1023
32g mol-1
= 6.02 x 1023
The number of oxygen atoms is 6.02 x 1023
STOICHIOMETRY OF REACTION
The calculation of the amounts (generally measured in moles or grams) of reactants and products involved in a chemical reaction is known as the stoichiometry of the reaction. In other words, the mole ratio in which reactants combine and products are formed gives the stoichiometry of the reactions.
From the stoichiometry of a given balanced chemical equation, the mass or volume of the reactant needed for the reaction or products formed can be calculated.
CALCULATION OF MASSES OF REACTANTS AND PRODUCTS
- Calculate the mass of solid product obtained when 16.8g of NaHCO3was heated strongly until there was no further change.
Solution:
The equation for the reaction is:
2NaHCO3(s) → Na2CO3(s) + H2O(g) CO2(g)
Molar mass of NaHCO3 = 23 + 12 + 16×3 = 84g mol-1
Molar mass of Na2CO3 = 23×2 +12+16×3 = 106g mol-1
From the equation:
2 moles NaHCO3 produces 1 mole Na2CO3
2x84g NaHCO3 produces 106g Na2CO3
16.8g NaHCO3 will produce Xg Na2CO3
Xg Na2CO3 = 106g x 16.8g
2x84g
=10.6g
Mass of solid product obtained = 10.6g
- Calculate the number of moles of CaCl2 that can be obtained from 25g of limestone [CaCO3] in the presence of excess acid.
Solution:
The equation for the reaction is:
CaCO3(s) + 2HCl → CaCl2(s) + H20(l) + CO2(g)
Number of moles = Reacting mass
Molar mass
Molar mass of CaCO3 = 40 + 12 + 16×3 = 100 gmol-1
Number of moles of CaCO3
= 25g
100 gmol-1
= 0.25 mole
From the equation of reaction,
1 mole CaCO3 yields 1 mole CaCl2
Therefore, 0.25 mole CaCO3 yielded 0.25 mole CaCl2.
ASSIGNMENT
In an experiment, 10cm3 of ethene [C2H4] was burnt in 50cm3 of oxygen.
- a) Which gas was supplied in excess? Calculate the volume of the excess gas remaining at the end of the reaction.
- b) Calculate the volume of CO2 gas produced