Chemical Reactions SS2 Chemistry Lesson Note
Download Lesson NoteTopic: Chemical Reactions
MEANING OF RATE OF REACTION
The rate of a chemical reaction is the number of moles of reactants converted or products formed per unit of time.
Usually, the rate of reaction is determined experimentally by measuring the change in concentration of one of the components in the reaction with time.
Thus,
Rate of reaction = change in concentration of reactant or product (mol/dm 3) ÷ Time taken for the change (seconds)
The unit of the rate of reaction is mol/dm-3s-1 or g dm-3s –1.
The rate of reaction can also be expressed as:
Rate of reaction = change in the number of mole or mass of a reactant or product ÷ Time taken for the change
Then the unit of rate is mols-1 or gs-1
EXAMPLE: When 0.5g of calcium trioxocarbonate (IV) was added to excess dilute hydrochloric acid, carbon (IV) oxide was evolved. The complete reaction took 5 minutes. What was the rate of reaction?
SOLUTION: Rate of reaction = change in mass of reactant ÷ Time taken for the change
= (0.5 – 0)g = 0.5
5×60 300
= 1.67 x 10-3 gs-1
WAYS OF MEASURING REACTION RATE
Concentration is one of the properties of a reaction that can change with time.
The following properties can also change with time and can thus be used to measure the rate of reaction.
- Decrease in mass of reaction system
- Volume of gaseous product
- Amount of precipitate formed
- Change in colour intensity
- Change in pH
- Change in total gas pressure
RATE CURVE
The rate curve is a graphical illustration of the rate of a reaction.
The graph below illustrates the rate curve

FEATURES OF RATE CURVE
- It passes through the origin. This is because there is no change in concentration or mass at the start of the reaction.
- It steeps at first, this is because the rate is fast at the beginning.
- It becomes less steep later. This is because the rate slows down.
- It finally becomes horizontal. This is because the reaction has reached the endpoints.
The following can be determined from the rate curve:
- Average rate of reaction
Average rate = total number of mole/mass involved Time taken
2. Rate at a particular instant during the reaction
Rate at instant = Gradient at a point on the curve
When the rate of reaction has a direct variation with concentration, then
Rate of reaction
= α[Concentration of [A]
R α [A]
R = k[A]
Where k is called the Rate Constant
COLLISION THEORY
The collision theory states that for a chemical reaction to occur the reactant particles must collide and they must collide with a certain minimum amount of energy known as activation energy.
Reacting particles are in continuous motion, thus they possess energy and they also collide with one another. Not all collisions result in chemical reactions. Collisions, which result in chemical reactions, are called effective collisions. The minimum amount of energy required by reacting particles for chemical reactions to occur is called activation energy. Activation energy is the energy barrier the reactants must overcome for the reaction to occur. It is the minimum energy required for bond breaking for a chemical reaction to occur.
Chemical reactions occur only when the energy of the colliding reactant particles is equal to or more than the activation energy. Activation energy must be equal to energy barriers also for a chemical reaction to occur.
Every reaction has its energy of activation. Reactions with low activation energy have a high rate of reaction and occur spontaneously. Reactions with high activation energy have a low rate of reaction and are not spontaneous.
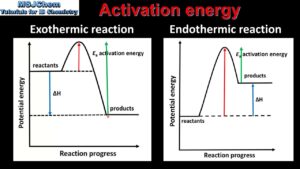
The graph below illustrates the concepts of the activation energy for endothermic and exothermic reactions.

From the graph, it can be seen that
- Both exothermic and endothermic reactions generally require an initial input of energy to overcome the activation energy barriers.
- Exothermic reactions once started proceed without any further external energy supply e.g. burning of kerosene.
- Endothermic reaction proceeds with a continuous supply of external energy e.g. cooking of rice.
FACTORS AFFECTING RATE OF REACTION
From the collision theory, it can be seen that rates of reaction depend on the following features.
- The energy of the particle.
- The frequency of collision of the reaction.
- The activation energy of the reaction.
These features of a chemical reaction are in turn affected by some factors, which can make them change and consequently affect the rate of reaction. These are factors that affect the rate of reactions. Some important ones are:
- Nature of reactants.
- Concentration/pressure (for gasses) of reactants.
- Surface area of reactants
- Temperature of reaction mixture
- Presence of light
- Presence of catalysts
To study the effect of any one of these factors on the rate of reaction all other factors must be kept constant.
- Effect Of Nature Of Reactants
If all other factors are kept constant, different substances will have different rates of reaction with dilute HCl, for example. When dilute HCl reacts with zinc, iron and gold under the same condition, hydrogen gas evolves fast with zinc, slowly with iron and no gas evolves with gold.
The difference in rate of reaction is due to the chemical nature of the elements as they naturally possess different amounts of energy content.
- Effect Of Concentration Of Reactants
The frequency of collision among particles is high when the particles are crowded in a small space, i.e. high concentration. This leads to highly effective collision and thus high rate of reaction. An increase or decrease in the concentration of the reactants will result in a corresponding increase or decrease in effective collisions of the reactants and hence the reaction rate.
- Effect Of Surface Area Of Reactants
This is a very important factor to be considered when a solid is involved in a chemical reaction. Lumped solids offer a small surface area of contact for reaction while powdered solids offer a large surface area for reaction. The rate of reaction is slow with lumped solids but high with powdered solids.
- Effect Of Temperature
Increasing the temperature of a system can lead to an increase in reaction rate in two ways. When heat is raised, energy in the form of heat is supplied to the reactant particles, so that:
i. The number of particles with energy equal to or greater than the activation energy increases.
ii. The velocity of all the reactant particles increases due to the greater kinetic energy, leading to a higher frequency of collision.
iii. As a result, the number of effective collisions increases and the reaction proceeds at a faster rate. Decreases in temperature lead to a decreased rate of reactions.
- Effect Of Light
Some reactions are influenced by light. The rate of reaction is high when the light’s intensity is high, low when the intensity is low and does not proceed at all in the absence of light. Such reactions are known as photochemical reactions. Examples of photochemical reactions
include:
i. Reaction between hydrogen and chlorine and
ii. Decomposition of hydrogen peroxide
iii. Reactions between methane and chlorine
iv. Photosynthesis in plant
v. Conversion of silver halides to grey metallic silver.
- Effect Of Catalyst
A Catalyst is a substance, which alters the rate of a reaction, but itself does not undergo any change at the end of the reaction.
A positive catalyst increases the rate of reaction by lowering the activation energy of the reaction whereas the one which increases the activation energy is known as a negative catalyst or an inhibitor.
The diagram below illustrates the energy profile for catalyzed and uncatalyzed exothermic and endothermic reactions:
ASSIGNMENT
- Define the rate of reaction b) State the collision theory
2a. Explain in terms of collision theory, how the rate of gaseous reaction is affected by an increase in pressure
- b) Give the reason why red-hot iron wool reacts more readily with oxygen than red-hot iron nail