Particulate Nature Of Matter SS1 Physics Lesson Note
Download Lesson NoteTopic: Particulate Nature Of Matter
Concept of Matter
The atomic theory of matter assumes that all matter is made up of tiny particles called atoms and that these are at all times in a rapid state of motion.
Simple Atomic Structure
Atoms are made up of particles, namely: protons, Electrons and neutrons. Protons and neutrons are found in a closely packed nucleus. A proton is positively charged, an electron is negatively charged and the neutron is neutral.
The electron revolves around the nucleus while the proton and neutron form the main nucleus at a relatively far distance from it. The number of protons in the nucleus equates to the number of electrons. The charge carried by a proton is positive (+ve) while the mass is 1 and the charge carried by electrons is negative (-ve) while the mass is 1/1840 mass of hydrogen. Neutron has no charge and the mass is 1.
- Matter
Matter is defined as anything that has weight and occupies space. Matter exists in three states, namely: Solid, Liquid and gaseous state. An example of a state of matter is Ice, a Liquid state of matter is water and a gaseous state of matter is steam. Other examples of solid are: brick, metal, concrete, and wood and liquid states are: Milk, oil, and gaseous states are oxygen, nitrogen, carbon dioxide, hydrogen, etc.
General Properties Of Matter
The properties of matter which are common to all substances are as follows:
- Matter is made up of tiny particles, molecules or atoms.
- Matter occupies space.
- Matter possesses inertia, mass or weight.
- Matter exerts pressure.
2. Properties of solids
The atomic theory of matter assumes that all matter is made up of tiny particles called atoms and that these are at all times in a rapid state of motion. In solids the molecules:
- Are arranged in regular patterns.
- Are tightly packed together.
- Are held together by strong forces of attraction called cohesive forces, and
- Are not free to move about, but merely vibrate about their mean position.
3. Properties of liquids
In liquids:
- The molecules are not arranged in any regular pattern.
- The molecules can slide past one another
- Cohesive forces between molecules, though present, are not as strong as in solids
- The molecules move at different speeds.
- Some of the faster molecules escape from the surface of the liquid giving rise to evaporation.
Properties of gases
In gases:
- The molecules move about rapidly and randomly.
- The molecules move at different speeds.
- The molecules are farther apart than in solids or liquids; and
- Cohesive forces are very small.
Note:
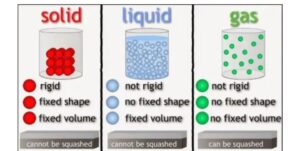
- Solids are rigid, have fixed shapes and volumes, and generally have higher densities than liquids and gases.
- A liquid has a definite volume but no definite shape. It takes on the shape of the container.
- A gas has no definite volume or shape. It will expand to fill the container.
- Molecules
Most substances cannot exist by themselves as individual atoms. They combine their atoms with themselves or with other atoms to form molecules.
A molecule is the smallest particle of a substance which can have a separate existence and still retain the properties of that substance.
Size of molecules:
To determine the size of molecules, oil is spread in drops over a surface of clean water in a container. Talcum or Lycopodium powder can be used to dust the surface of the water, to show clearly where exactly the oil is i.e. the powdery surface will be drawn back as the oil spreads.
To determine the size of a molecule
Taking the volume of oil as A₁cm³ and the area of the surface covered by the oil as A2 cm².
The volume of oil drop = volume of oil from, i.e.
4/3π³ = πR2h
Where h is the height of the oil form. The thickness of one molecule, R and r are the radii of the oil dropped and oil formed. 4/3π³ = πR2h
Therefore the thickness of one molecule is referred to as h.
h = (4/3π³) / (πρ²)
The thickness is between 107cm and 109cm.
Diffusion
Diffusion is the process by which different forms of matter (fluids) mix intimately with one another owing to the kinetic nature of their particles or molecules.
Factors Affecting Diffusion
- The density of the substance
- The temperature of the substance
- Pressure
- Concentration
- Mass
Osmosis
Osmosis is the movement of water molecules from a region of higher concentration to a region of lower concentration through a semi-permeable membrane.
Osmosis is not limited to sugar solutions. It occurs whenever any solution is separated from more of its solvent by a semi-permeable membrane, or when a dilute solution is separated from a more concentrated one.
NOTE:
- Plants obtain moisture by the process of Osmosis across the semi-permeable membrane of the root cells.
- The human body also contains semi-permeable membranes which play an important part in the transfer of liquid from one part to another.
Brownian Motion
Brownian motion or movement is the evidence that molecules exist in matter and that the molecules are continually in motion. This makes us look considerably at the smoke. When a burning fire is kindled or lightly, smoke is seen to move upward into the sky. The smoke is seen to move upward into the sky. The smoke moves in a way or direction that is not straight but keeps changing. The non-linear direction of the smoke is evidence of Brownian motion.
Brownian’s conclusion, therefore, was that:
- Molecules are continually in motion.
- Molecules exist.