Carbon And Its Compounds I SS1 Chemistry Lesson Note
Download Lesson NoteTopic: Carbon And Its Compounds I
OCCURRENCE OF CARBON
- It occurs naturally as diamond and graphite.
- It occurs in an impure form as coal.
- It occurs in the combined state as petroleum, wood and natural gases.
- It occurs in minerals such as limestone (CaCO3) and dolomite (MgCO3)
- It occurs in the atmosphere (air) as CO2
- It is an essential constituent of all forms of plant and animal life.
ALLOTROPES OF CARBON
Allotropy is the phenomenon whereby an element exists in two or more different forms in the same physical state. The different forms of the elements are known as allotropes. They have the same chemical properties but different physical properties.
Carbon exists in several allotropic forms:
(1). Crystalline Allotropes e.g Diamond and graphite
(2). Non-crystalline Allotropes/Amorphous carbon e.g coal, charcoal, coke, lampblack and carbon black (soot)
CRYSTALLINE ALLOTROPES OF CARBON
- Diamond: Diamond is the purest form of carbon. The diamond crystal is octahedral in shape. It is a giant molecule in which the carbon atoms are closely packed and held together by strong covalent bonds.
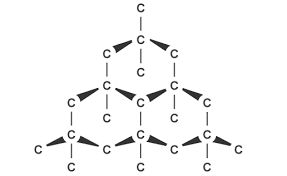
Basic Tetrahedral Shape in Diamond Crystals
PROPERTIES OF DIAMOND
a) Diamond is extremely hard and strong with a high melting point because of its strong covalent bond. Diamond is the hardest substance known in the world.
b) It has a high density because of its compactness of crystal.
c) It is very resistant to chemical action and temperature because all four valence electrons are saturated and bonded.
d) It is a non-conductor of electricity because there are no free valence electrons in the crystal lattice.
e) Transparent and highly refractive, hence it is used as a jewel and sparkling substance.
USES
a) They are used industrially in drills for mining since they are dense and hard.
b) They are used to sharpen very hard tools.
c) They are used for cutting glass and metals.
d) They are also used as pivot supports in precision instruments and as dies for drawing wires
e) It is valuable in making jewellery (i.e. its high refractive index and dispersion power give it a sparkling brilliance when it is cut and polished).
NOTE:
Artificial diamonds: They are made by subjecting graphite to a very high temperature and pressure for several hours in the presence of a nickel or rhodium catalyst.
GRAPHITE: The graphite crystal is hexagonal. The carbon atoms in graphite form flat layers. These layers are arranged in parallel, one above the other to form a crystal lattice.
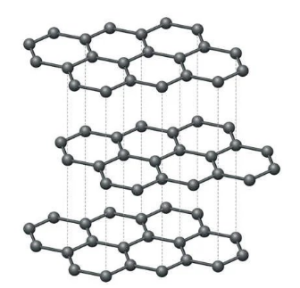
PROPERTIES OF GRAPHITE
a) Graphite is soft and slippery because of weak forces holding its layers. Each layer can slide over one another. Hence, graphite acts as a lubricant.
b) It is less dense and prone to chemical attack due to its open structures in layers.
c) It is a good conductor of electricity because of the presence of free delocalized electrons (mobile electrons) in the crystal lattice.
d) It is inert and used to absorb radiation in nuclear stations in atomic piles.
USES
a) It is usually used on bicycle chains and for the bearings of some motor cars.
b) It is used as a non-greasy lubricant (i.e. combining it with oil makes a high-temperature lubricant).
c) It is used as electrodes in electroplating and dry cells (since it is a good conductor of electricity and relatively inert).
d) Graphite can be used to make a non-conductor conductive by coating with it.
e) It is used to line crucibles for making high-grade steel and other alloys (since it can withstand high temperatures).
f) It is used in making lead pencils i.e. combining it with clay makes lead in pencils.
g) It is used as a black pigment in paints.
h) It is used as a neutron moderator in atomic piles.
INDUSTRIAL PREPARATION OF GRAPHITE
Graphite is produced industrially by heating coke in an electric furnace to a very high temperature for about 20 to 30 hours. This process is called the Acheson process. The Acheson process is a process of producing graphite from coke at high temperatures. Air is excluded by covering the coke with sand. The graphite produced is very pure and free from grit.
DIFFERENCES IN PROPERTIES BETWEEN GRAPHITE AND DIAMOND
| S/N | GRAPHITE | DIAMOND |
| 1 | It has a density of 2.3gcm-3 | It has a density of 3.5gcm-3 |
| 2 | It is a black, opaque solid | It is a colourless, transparent solid |
| 3 | It is very soft, marks paper | It is the hardest known substance. |
| 4 | It is a good conductor of electricity | It is a non-conductor of electricity |
| 5 | Attacked by potassium trioxochlorate (v) and trioxonitrate (v) acid together. | Not attacked by these reagents. |
Note: Diamond is transparent to X-rays while glass is almost opaque.