Particulate Nature Of Matter SS1 Chemistry Lesson Note
Download Lesson NoteTopic: Particulate Nature Of Matter
NATURE OF MATTER
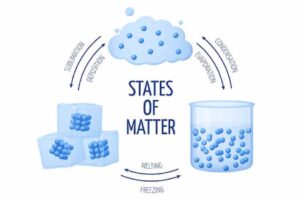
The matter is anything that has weight and occupies space. It exists in three states namely: solid, liquid and gas.
The fundamental difference between the three states of matter depends on the degree of movement of the particles they are made of.
- SOLID STATE
The particles of a solid are tightly packed and held together by a strong electrostatic force.
The particles only vibrate to and fro about an equilibrium or a fixed position. They have a definite shape and volume and are very difficult to compress
- LIQUID STATE
The forces of attraction between molecules of liquids are weak compared to that of solids. Hence they have slight movements. This is why liquids can flow. They have definite volume but not definite shape.
- GASEOUS STATE
As a result of the distance between the molecules of gases, the cohesive forces between them are very negligible and so they move randomly. Gases have no definite shape or volume. They assume the shape of the containing vessel.
COMPARISON BETWEEN SOLID, LIQUID AND GAS
| S/N | SOLID | LIQUID | GAS |
| 1 | Fixed mass | Fixed mass | Fixed mass |
| 2 | Very dense | Less dense | Least dense |
| 3 | Definite shape | Shapeless | Shapeless |
| 4 | Definite volume | Definite volume | No volume |
| 5 | Incompressible | Incompressible | Compressible |
| 6 | Particles vibrate | Particles vibrate | Particles move |
TYPES OF CHANGES
Whenever a given substance is heated, its state changes. There are two types of changes: physical and chemical.
- PHYSICAL CHANGE:
A physical change is a change which is easily reversible and in which no new substances are formed. Examples are:
- Dissolution of common salt
- Changes in states of matter such as melting of solids, freezing of liquids, evaporation of liquids, liquefaction of gases to solids, and sublimation of solids.
- Magnetization and demagnetization of iron nails.
- Separation of mixture by evaporation, distillation, fractional distillation etc.
2. CHEMICAL CHANGE:
A chemical change is a change which is not easily reversible and in which new substances are formed.
Examples of chemical change are:
- Rusting of iron/metals.
- Dissolution of metals and limestone in acids.
- Fermentation and decay of substances.
- Changes in electrochemical cells.
- The addition of water to quick lime.
- Burning of materials.
COMPARISON BETWEEN PHYSICAL AND CHEMICAL CHANGES
| S/N | Physical Change | Chemical Change |
| 1 | Easy to reverse | Difficult to reverse |
| 2 | No new substances are formed | New substances are always formed |
| 3 | Very little energy changes take place | There are often large heat change |
| 4 | No change in mass | The new substances formed have different masses but the total mass remains unchanged |
ASSIGNMENT
- Give two differences between physical and chemical changes.
- Give three processes, which involve a physical change
ATOMS AND MOLECULES
Matter is made up of discrete particles. The main ones are atoms, molecules, and ions.
An atom is the smallest part of an element which can take part in a chemical reaction.
A molecule is the smallest particle of a substance that can exist alone and still retain the chemical properties of that substance. Molecules are made up of atoms.
The atomicity of an element is the number of atoms in one molecule of the element.
We have monatomic, diatomic and triatomic for those elements that contain one atom, two atoms and three atoms respectively in their molecules.
Examples:
| S/N | Element | Atomicity |
| 1 | Hydrogen | Diatomic |
| 2 | Oxygen | Diatomic |
| 3 | Nitrogen | Diatomic |
| 4 | Neon | Monoatomic |
| 5 | Helium | Monoatomic |
| 6 | Argon | Monoatomic |
An ion is an atom or group of atoms which carries an electric charge. Such groups of atoms that carry either a positive or negative charge are called RADICALS.
An acid radical is thus a small group of atoms carrying a negative charge that keeps its identity. Examples include S042-, N03- e.t.c.
Generally, ions are grouped as cations and anions. Cations are positively charged ions e.g. Ca2+, Na+, NH4+ etc.
Anions are negatively charged ions e.g……. C032-, S042-, Cl-, OH-, etc.
DALTON’S ATOMIC THEORY
John Dalton, British Physicist and Chemist (1808) proposed the atomic theory thus:
- All elements are made up of small indivisible particles called atoms.
- Atoms can neither be created nor destroyed in any chemical reaction.
- Atoms of the same elements are exactly alike in aspect and are different from atoms of all other elements.
- Atoms of different elements can combine in simple whole-number ratios to form compounds.
- All chemical changes result from the combination or separation of atoms
MODIFICATIONS OF DALTONS ATOMIC THEORY
Due to discoveries in the twentieth century, Dalton’s atomic theory cannot hold in its entirety. There is a need for its modification.
The first statement was proved wrong by Rutherford’s discovery of protons, electrons and neutrons as constituents of the atom. An atom is not an indivisible solid piece.
The second statement still holds good for ordinary chemical reactions. During nuclear reactions, however, the nucleus can be broken into simpler atoms giving out large amounts of heat (nuclear fission). This destroys the atoms involved.
The discovery of isotopes makes the third statement unacceptable. Chlorine for example has two atoms with different nucleus content and hence different relative atomic masses although the same proton numbers.
The fourth statement is true only for inorganic compounds which contain a few atoms per molecule. Carbon forms very large organic molecules such as proteins, starch and fats which contain thousands of atoms.
ASSIGNMENT
- Give any two postulates of the Dalton atomic theory.
- Differentiate an atom from a molecule.
- How will an atom become an ion?