Chemical Combinations SS1 Chemistry Lesson Note
Download Lesson NoteTopic: Chemical Combinations
ELECTROVALENT (IONIC) BOND
An electrovalent bond is characterised by the transfer of electrons from metallic atoms to non-metallic atoms during the reaction. The metallic atom that donates electrons becomes positively charged while the non-metallic atom that accepts electrons becomes negatively charged. The strong electrostatic attraction that holds the oppositely charged ions together is called an ionic bond.

ELECTRON DOT REPRESENTATION OF THE FORMATION OF IONIC COMPOUNDS
Formation of sodium chloride and calcium oxide
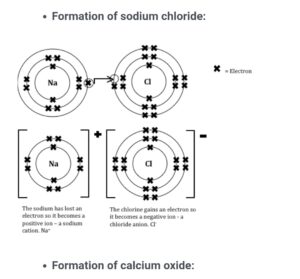
PROPERTIES OF SOME IONIC COMPOUNDS
- They are solids at room temperature.
- They contain oppositely charged ions.
- They readily dissolve in water and other polar solvents like ethanol.
- They have high melting and boiling points.
- They are good conductors of electricity when in molten or in aqueous form.
- COVALENT BOND
This involves the sharing of a pair of electrons between two reacting atoms. The shared electrons are each contributed by the reacting atoms and are called shared pairs. A shared pair of electrons in a covalent bond is represented by a horizontal line
(—-) between the two atoms
ELECTRON DOT REPRESENTATION OF THE FORMATION OF COVALENT COMPOUNDS
Formation of hydrogen molecule and carbon (iv) oxide
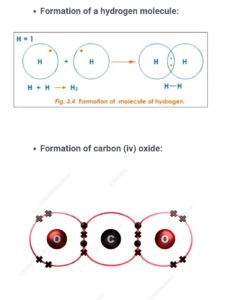
PROPERTIES OF COVALENT COMPOUNDS
- They consist of molecules with a definite shape.
- They are gases or volatile liquids.
- They readily dissolve in non-polar organic solvents
- They have low melting and boiling points
- They do not conduct electricity because the molecules do not contain charged particles.
COORDINATE COVALENT (DATIVE) BOND
In a coordinate covalent bond, the shared pair of electrons is supplied by one of the combining atoms. A coordinate covalent bond is often formed in molecules that have a lone pair of electrons, i.e. a pair of electrons not directly concerned with an existing bond.
ELECTRON DOT REPRESENTATION TO SHOW FORMATION OF DATIVE BOND
Formation of hydroxonium and ammonium ion (H3O+)

Compounds containing coordinate covalent bonds are similar in properties to purely covalent compounds. Both do not conduct electricity, but the presence of a coordinate covalent bond tends to make a compound less volatile.
HYDROGEN BOND
A hydrogen bond is a dipole-dipole intermolecular force of attraction which exists when hydrogen is covalently bonded to a highly electronegative element of small atomic size. The electronegative element can be N, O, F, Cl, Br or I.
The highly electronegative element has a very strong affinity for electrons. Hence, they attract the shared pair of electrons in the covalent bond toward themselves, resulting in the formation of a dipole which leaves a partial positive charge on the hydrogen atom and a partial negative charge on the electronegative atom. An electrostatic attraction between two dipoles is set up when the positive pole of one molecule attracts the negative pole of the other. This attractive force is known as a hydrogen bond.
IMPORTANCE OF HYDROGEN BOND
- It accounts for the solubilities of some compounds containing O, N and F in certain hydrogen-containing solvents such as water
- The crystalline shape of solid water (ice) is due to hydrogen bonds.
METALLIC BOND
Metal atoms are held together in a solid crystal lattice by metallic bonds. each metallic atom contributes its outer (valence) electron to the electron cloud, thus becoming positively charged. The resulting positively charged metallic ions tend to repel each other but are held together by the moving electron cloud and overlapping residual electron orbits. Thus, a metallic bond is a force of attraction between the positive metal ions and the free mobile electrons.
VAN DER WAALS’ FORCES
The attractive forces which make it possible for non-polar molecules like nitrogen and CO2 molecules to form liquid and solid is called van der Waals’ force. This force though very weak when compared to ionic and covalent bonds is important in the liquefaction of gases and in the formation of molecular lattices as in iodine and naphthalene crystals.
STATES OF MATTER
The three states of matter: solid, liquid and gaseous states can be distinguished by the motion of the particles they are made of and the attractive force between their particles.
CHANGE OF STATE
- MELTING
Melting is the physical process where a substance changes from a solid to a liquid. When a solid is heated, the particles acquire greater kinetic energy and move violently. A point is reached when the forces of vibration overcome the cohesive forces holding the solid particles together and the crystalline structure collapses. The particles are no longer held in fixed positions but are free to move about and the liquid state is reached. The temperature at which this occurs is called the melting point of the solid.
- BOILING
When a liquid is heated, the rate of evaporation increases and the value of the saturated vapour pressure equals the prevailing atmospheric pressure. When this happens, the liquid is said to boil and the temperature at which this happens is known as the boiling point of the liquid.
The boiling point of a liquid changes with a change in atmospheric pressure. If the pressure is raised, the boiling point will increase and if the pressure is lowered the boiling point will decrease. Also, the presence of impurities increases the boiling point of a liquid.
- EVAPORATION
Evaporation is the process of vapourization of liquids at all temperatures. When the surface of a liquid is exposed, the molecules near the surface of the liquid will acquire extra kinetic energy, large enough to enable them to break away from the cohesive force binding them to the neighbouring particles. Once free, they escape from the liquid surface to become molecules in the vapour state.
Evaporation results in a decrease in the volume of liquid and lowers the temperature of the liquid, therefore it causes cooling. Also, it occurs at all temperatures but increases with an increase in temperature. In addition, it is slower in electrovalent liquids than in covalent liquids.
DIFFERENCES BETWEEN EVAPORATION AND BOILING
- Evaporation takes place at the surface of the liquid while boiling involves the entire volume of the liquid
- Evaporation takes place at all temperatures but boiling takes place at a fixed temperature
CONDENSATION AND FREEZING
Condensation is a process whereby a vapour loses some of its kinetic energy to a colder body and changes into a liquid state.
When a liquid cools, it loses heat energy to its surroundings, causing its temperature to drop. If the cooling continues, the temperature of the liquid keeps dropping until it reaches the freezing point of the liquid. At this temperature, the liquid changes into a solid.
KINETIC THEORY OF GASES
The theory postulates the following for an ideal or perfect gas:
- Gas molecules are in a constant, rapid, straight motion, colliding with one another and with the walls of the container.
- The collision of gas molecules is perfectly elastic.
- The total volume of the gas molecule is negligible compared to the volume of the container.
- The force of attraction between the gas molecules is negligible.
- The average kinetic energy of the molecule is a measure of the temperature of the gas molecules.
PHENOMENA SUPPORTING THE KINETIC THEORY OF GASES
- Brownian motion: This is the constant, irregular movement of particles in a liquid or gas. It shows that gas molecules are in constant motion.
- Diffusion: Diffusion is the movement of particles from a region of higher concentration to a lower concentration. Diffusion is common in gases and it results from the random movement of particles of a gas.