Kinetic Molecular Theory: Boiling And Evaporation JSS2 Basic Science Lesson Note
Download Lesson NoteTopic: Kinetic Molecular Theory: Boiling And Evaporation
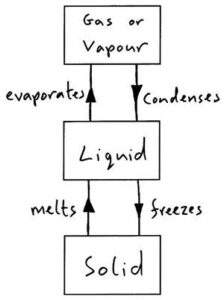
Kinetic molecular theory is useful in describing the properties of solids, liquids, and gases at the molecular level. The kinetic theory of matter assumes that matter is made up of tiny particles such as atoms, molecules, and ions that are continually moving and therefore possess kinetic energy. The more heat you give these particles, the faster their movement will be. Hence, an increase in temperature causes an increase in the average kinetic energy of the particles. Matter exists in three states which are Solid, Liquid, and Gas.
Solids
The particles of a solid will possess only a small amount of kinetic energy. The particles of a solid are always in motion, however, the motion will be so minuscule that we can say that they are simply vibrating in position. Thus, the particles of a solid will have a very ordered structure. Solids have a definite shape, and a definite volume and are not easily compressed.
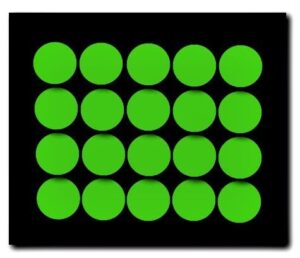
The particles of a solid are vibrating in a very ordered arrangement
Liquids
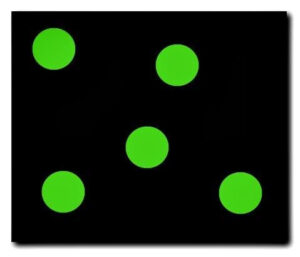
The particles of a liquid possess a greater amount of kinetic energy than the particles of a solid. Thus, the particles of liquid will not be in an ordered arrangement and will take on the shape of the container that they are placed in. These particles will possess enough energy to flow throughout a container and past one another. They do not have enough energy to escape the attraction they have for each other and as a result, will remain loosely connected. Liquids have an indefinite shape, and a definite volume and are not easily compressed.
The particles of a liquid are flowing in a loosely connected arrangement.
Gases
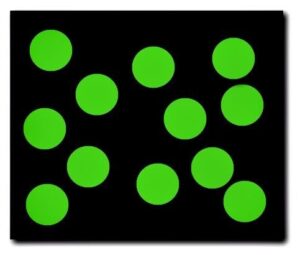
The particles of a gas possess a very large amount of kinetic energy and move very rapidly and randomly. The particles of a gas are moving so fast that they have no attraction to one another. As a result of this kinetic energy, the particles of a gas will have large empty spaces between them. Just as with liquids, gases will take on the shape of the container that they are placed in. Gases have an indefinite shape, and an indefinite volume and are easily compressed (due to the large empty spaces between particles)
The particles of a gas are moving very rapidly and randomly.