Soaps SS3 Chemistry Lesson Note
Download Lesson NoteTopic: Soaps
SPECIFIC OBJECTIVES: At the end of the lesson, pupils should be able to
- describe the preparation of soap.
- Explain the cleansing action of soap.
INSTRUCTIONAL TECHNIQUES:
- Identification,
- explanation,
- questions and answers,
- demonstration,
- videos from the source
INSTRUCTIONAL MATERIALS:
- Videos,
- loudspeaker,
- textbook,
- pictures,
- Vegetable oil,
- Caustic soda or potash,
- Wood ash,
- Containers/reaction vessels.
NOTE
Preparation of soap
The preparation of soap involves the saponification reaction, where fats or oils react with a strong base (alkali), such as sodium hydroxide (NaOH) or potassium hydroxide (KOH), to produce soap and glycerol. Here is a simplified equation:
Triglyceride + 3NaOH Glycerol + 3 Soap Molecules
For a more specific example, let’s consider the saponification of a common fat, such as coconut oil, which contains a mixture of fatty acids:
Coconut Oil (C12H24O2) + 3NaOH Glycerol(C3H8O3) + 3 Sodium Fatty Acid Salts(Soap)
In this reaction, coconut oil, which is a triglyceride, reacts with three molecules of sodium hydroxide to produce glycerol and three molecules of sodium fatty acid salts, which are soap molecules.
NOTE: The soap molecules have a hydrophilic (water-attracting) head and a hydrophobic (water-repelling) tail, allowing them to interact with both water and grease. This makes soap an effective cleaning agent, as it can emulsify and lift away oily or greasy substances in the presence of water.
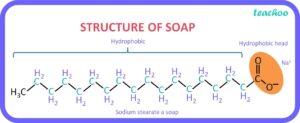
Structure of Soap
The structure of soap molecules consists of two main parts: a hydrophilic (water-attracting) “head” and a hydrophobic (water-repelling) “tail.” This dual nature allows soap molecules to interact with both water and oils, making them effective in cleaning. The typical structure of a soap molecule is illustrated below:
The action of soap as an emulsifying agent
Soap acts as an emulsifying agent due to its unique molecular structure. The hydrophilic (water-attracting) and hydrophobic (water-repelling) parts of soap molecules enable them to interact simultaneously with both water and oily substances. The steps describe how soap functions as an emulsifying agent:
- Hydrophilic Head Interaction:
- The hydrophilic head of the soap molecule is attracted to water molecules, allowing it to be soluble in water.
2. Hydrophobic Tail Interaction:
- The long hydrophobic tail of the soap molecule is repelled by water but is attracted to oils and grease.
- Formation of Micelles:
- When soap is added to water containing oils or grease, the soap molecules arrange themselves into structures called micelles.
- In a micelle, the hydrophobic tails cluster together in the centre, shielding themselves from water, while the hydrophilic heads face outward, interacting with water molecules.
3. Encapsulation of Oil:
- The hydrophobic tails of the soap molecules embed into the oil or grease, forming a core within the micelle.
4. Dispersion in Water:
- The hydrophilic heads on the outer surface of the micelle interact with water, keeping the entire structure suspended in the water.
NOTE: This emulsifying action allows soap to surround and disperse oily substances in water, forming stable emulsions. These emulsions can then be easily rinsed away, carrying the trapped grease with them. This property makes soap an effective cleaner for removing oils, fats, and other hydrophobic substances from surfaces.
EVALUATION:
- Discuss the preparation of soap.
- Describe the functions of soap as an emulsifying agent
CLASSWORK: As in evaluation
CONCLUSION: The teacher commends the students positively