Qualitative Analysis SS3 Chemistry Lesson Note
Download Lesson NoteTopic: Qualitative Analysis
SPECIFIC OBJECTIVES: At the end of the lesson, pupils should be able to
- identify ions in solution.
- Test for simple gases like NH3, NO3, Oxygen etc
INSTRUCTIONAL TECHNIQUES:
- Identification,
- explanation,
- questions and answers,
- demonstration,
- videos from the source
INSTRUCTIONAL MATERIALS:
- Videos,
- loudspeaker,
- textbook,
- relevant salts,
- acids,
- bases,
- starch, etc.
NOTE
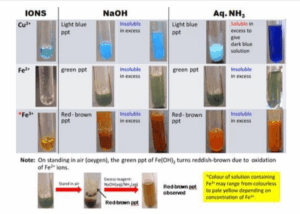
Qualitative analysis involves the examination of colour, flame test, the effect of heat and confirmatory test for cation and anions.
Cations are metallic ions e,g. Ca<sup>2+</sup>, Pb<sup>2+</sup>, Al<sup>3+</sup>, Cu<sup>2+</sup>, Fe<sup>2+</sup>, Fe<sup>3+</sup>, etc
Rules in qualitative analysis
- Your test solution should not be diluted too much
- Use only a small quantity of reagents
Examination of colour and physical state of the specimen
Substance colour physical state
- Sulphur yellow solid
- Copper(ii) oxide black solid
- Iodine dark brown solid
- Nitrogen iv oxide reddish-brown gas
- Mercury white liquid
Flame test
- Deep green colour of flame indicates the presence of copper
- Deep yellow colour indicates the presence of sodium
- Brick red indicates calcium.
Evaluation
- State ten (10) examples of cations
- Give the colour of the following substance.
- Distilled water
- Iron filling
- Manganese(iv) oxide
- Benzoic acid.
Test for cations
The Cations are Ca<sup>2+</sup>, Zn<sup>2+</sup>, Al<sup>3+</sup>, Pb<sup>2+</sup>, Fe<sup>2+</sup>, Fe<sup>3+</sup>, Cu<sup>2+</sup>, NH<sub>4</sub><sup>+</sup>
Alkaline gas
Gas + con HCI It gives white fumes with conc HCI NH<sub>3</sub> gas from NH<sub>4</sub><sup>+</sup>
General evaluation
- Give the common reagents used for the confirmatory test for cations.
- State the colour of the solution when the sample that contains the following dissolves in water: a. Fe <sup>2+</sup> b. Cu <sup>2+</sup> c. Fe <sup>3+</sup>
Weekend assignment
- The following gives a white gelatinous precipitate in NaOH except? (a) Al <sup>3+</sup> (b) pb<sup>2+</sup> (C) CU<sup>2+</sup> (d) Zn<sup>2+</sup>
- One of the following gives a ‘pop’ sound when in contact with a lighted splint. (a) O<sub>2</sub> (b) H<sub>2</sub> (c) NH<sub>3</sub> (d) CO<sub>2</sub>
- Example of cations that give gas during their confirmatory test is (a) SO<sub>3</sub><sup>2-</sup> (b) SO<sub>4</sub><sup>2-</sup> (c) CO<sub>3</sub><sup>2-</sup> (d) NH<sub>4</sub><sup>+</sup>
- Deep green colour of the flame indicates the presence of (a) sodium (b) calcium (c) copper (d) iron
- The only alkaline gas that changes moist red litmus paper to blue is (a)NH<sub>3</sub> (b) HCl (c) NaOH (d) NH<sub>4</sub>OH
Theory
- Explain the confirmatory test for the following cations
- State the flame test for the following: a. Calcium b. Copper c. sodium d. iron
Reading assignment
- School Chemistry by O.Y Ababio pages 165-183
- Practical Chemistry by R. I. Makanjuola pages 31-36.
- Practical Chemistry for Schools and Colleges by Godwin O. Ojokuku pages 30-98.