Shapes and Molecules SS3 Chemistry Lesson Note
Download Lesson NoteTopic: Shapes and Molecules
CONTENT
- Covalent Molecules e.g methane, diamond, crystalline solid – their network structure e.g diamond.
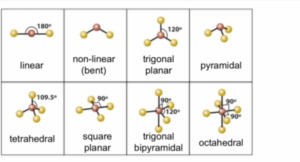
Simple molecules and their shapes
The factors that are responsible for the shape of simple covalent molecules are
- sharing of an electron that leads to the overlapping of two atomic orbital
- the central atom and its valence shell electron.
Covalent molecules
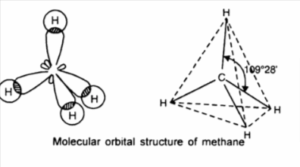
Let’s consider methane as an example (CH<sub>4</sub>).
The central atom is carbon with an electronic configuration of 1S<sup>2</sup>2S<sup>2</sup>2P<sup>2</sup> which can be spined as
During the bond formation between carbon and hydrogen, one electron is promoted from 2S to 2P². That is IS²2S¹2P¹x2P¹y2P¹ or
In the molecule of methane, the carbon atom has four bond pairs of electrons in its valence shell (the octet rule is obeyed).
The C-H bond in methane is identical. If the 2S and three 2P orbitals are hybridised to form four new orbitals which are identical, these new hybrid orbitals are called SP³. That is one S and three P orbitals are combined. The electron is negatively charged and they move to the corners of a regular tetrahedron. This carbon lies at the apices of the tetrahedron.
Shape of methane
Evaluation
- State two (2) factors that determine the shape of a simple covalent molecule
- Draw the shape of methane and explain its formation
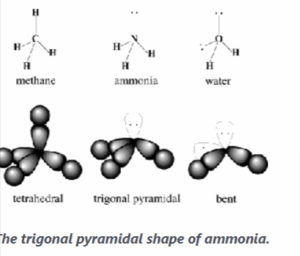
Ammonia
In ammonia, NH<sub>3</sub>, the central atom is Nitrogen with configuration 1S<sup>2</sup>2S<sup>2</sup>P<sup>3</sup> or
The three unpaired electrons in the 2P from the covalent bond with an election of the hydrogen atom. It remains one lone pair in the outermost valence shell of nitrogen and the octet rule is satisfied.
The electron clouds of 4 pairs of electrons spaced out but not of the same shape as methane because ammonia contains one lone pair of electrons. This gives ammonia a trigonal pyramidal shape.
Shape of Ammonia
Crystalline solids
- The crystalline solids have a definite geometric shape
- The relative sizes of the particles if they are different.
- The shape of the crystal depends on the force of attraction between the particles whether the particles are the same or different.
There are 3 types of unit cell crystals based on cubic structure.
- Simple cubic: the particles are placed one at each corner of the cube
- Face-centred cubic: the particles are located at each corner and one in the centre of each face of the cube.
- Body-centred cubic: there is a particle at each corner and one at the centre of the cube.
Types of crystalline solids
Crystals can be grouped according to the chemical nature of their particles
- covalent crystalline solid
- ionic crystalline solid
- molecular crystalline solid
- metallic crystalline solid.
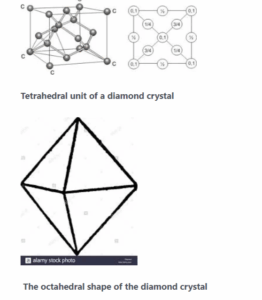
Covalent crystalline solid:
The best example is a chemical crystal which is octahedral in shape. The crystal lattice is built from a basic three-dimensional tetrahedral unit cell. The carbon atom is linked to four other carbon atoms by covalent bonds which are directed towards the apices of a regular tetrahedron.
Thus, the unit cell is repeated severally to form a giant three-dimensional molecule.
Evaluation
- Explain the crystalline solid
- Draw the structure of
- ammonia
- the octahedral shape of a diamond
Ionic solids
Examples are NaCl and CuSO4 crystals. The shape of these crystals is determined according to how positive and negative ions are arranged, and according to the sizes and changes of the ions.
Molecular crystals
There the molecules are arranged in regular patterns to give lattices. The molecules are held together by weak intermolecular forces e.g. Vander Waals force, hydrogen bond, dipole-dipole.
Examples of the molecules are Naphthalene, iodine and dry ice crystal.
Metallic solid
The metallic particles are held together in a crystal lattice of a closely – packed sphere.
The strength of the metallic bonds varies among different metals e.g. iron is stronger than sodium and potassium.
General evaluation
- What are the types of attractive forces present in each of the following substances at room temperature and pressure?
- Methane
- Argon
- Diamond
- Water
- Aluminium
Reading assignment
- New School Chemistry by O.Y. Ababio pages 286-294.
Weekend assignment
- A lone pair of electrons is found in (a) ammonia (b) methane (c) water (d) carbon(iv) oxide
- Examples of covalent molecules with linear shapes are (a) oxygen (b) hydrogen (c) water (d) hydrogen chloride
- Example of a compound with double bonds is (a) water (b) carbon(iv) oxide (c) methane (d) ammonia
- The following are types of crystalline solids except (a) covalent (b) ionic (c) metallic (d) methane
- The unit cells based on the cubic structure are the following except (a) simple cubic (b) complex cubic (c) body-centred cubic (d)face-centred cubic
Theory
- State three (3) examples of crystalline solids with their shape
- Explain the following simple covalent molecules and draw their shape (a) methane (b) Water (c) carbon (iv) oxide.