Non Metals: Oxgyen SS2 Chemistry Lesson Note
Download Lesson NoteTopic: Non Metals: Oxgyen
Oxygen is the most abundant element on earth. It constitutes 21% of the volume of atmospheric air.
Occurrence: It occurs as free elements in nature and combined states.
GENERAL PROPERTIES OF THE OXYGEN FAMILY
Elements in group VI include Oxygen (O), Sulphur (S), Selenium (Se), Tellurium (Te), and Polonium (Po) Their properties are as follows:
- They are non-metals and exist as solids at room temperature except for oxygen.
- They are electron acceptors and oxidizing in nature.
- They do not react with water in any form. But oxygen and sulphur combine directly with hydrogen to yield water and hydrogen sulphide respectively.
ELECTRONIC STRUCTURE AND BONDING IN OXYGEN
Oxygen has an atomic number of 8; hence its electronic configuration is 1s²2s²2p⁴. This shows that oxygen needs two electrons to attain an octet configuration.
The oxygen atom has six valence electrons and can acquire a stable octet configuration by:
- Accepting two electrons from electropositive elements like metals to form a negative oxide ion, O2-.
Example:
Ca2+ + O2- → CaO
Entering into covalent bond formation with non-metals by covalently sharing two out of its six outer electrons.
2. Forming covalent bonds with itself.
LABORATORY PREPARATION OF OXYGEN
- By the thermal decomposition of potassium trioxochlorate (V) in the presence of MnO2 as a catalyst
2KClO3(s)heat > 2KCl2(s) + 3O2(g)
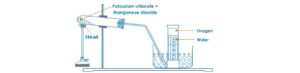
- By decomposition of hydrogen peroxide, H2O2 in the presence of MnO2 as a catalyst
2H2O2(aq) heat > 2H2O(l) + O2(g)
Hydrogen peroxide also reacts with acidified KMnO4 in the cold to produce oxygen
5H2O2(aq) + 2KMnO4(aq) + 3H2SO4(aq) > K2SO4(aq) + 2MnSO4(aq) + 8H2O(l) + 502(g)
NOTE: If the gas is required to be dry, it is passed through anhydrous calcium chloride or concentrated tetraoxosulphate (VI) acid and collected over mercury.
INDUSTRIAL PREPARATION
- Electrolysis of water
i. Fractional distillation of liquid air: This preparation involves two main processes:
- a) Liquefaction of air
Air is first passed through caustic Soda, NaOH(aq) to remove CO2. It is then subjected to a series of compressions, expansions and cooling until liquid air is obtained at -200oC.
b) Fractional distillation of liquid air
The liquid air is led to a fractional distillation column. On distillation, nitrogen with a lower boiling point of -1960C evolved first, leaving behind liquid oxygen. Further heating converts the liquid oxygen to a gas at -1830C
PHYSICAL PROPERTIES
- It is a colourless, odourless and tasteless diatomic gas
- It is neutral to litmus
- It is slightly soluble in water
- Gaseous oxygen is denser than air.
- Gaseous oxygen liquefies at -1830C.
CHEMICAL PROPERTIES
- Reaction with metals: Oxygen combines directly with most metals to form basic oxides
2Ca + O2 > 2CaO
4K + O2 > K2O
The oxides of very electropositive metals, K, Na, and Ca dissolve in water to form alkalis
2 K2O + 2 H2O > 4KOH
- Reaction with non-metals: Non-metals burn in oxygen to acidic oxides. These are known as acid anhydrides as they dissolve in water to form acids.
S(s) + O2(g) > SO2(g)
SO2(g) + H2O(l) > H2SO3(aq)
P4(s) + O2(g) > P4O6(g)
P4O6 + H2O(l) > 4H3PO3(aq)
- Most hydrocarbons and compounds of carbon, hydrogen and oxygen burn in oxygen to give CO2 and H2O
C2H5OH(l) + 3O4(g) > 2CO2(g) + 3H2O(l)
USES OF OXYGEN
- It is used in oxy-ethylene flame
- It is required for respiration
- It is used in the steel industry for the removal of C, S and P impurities from pig iron
- Liquid oxygen and fuel are used as propellant for space rockets
- Oxygen is used in the manufacture of tetraoxosulphate (VI), trioxonitrate (VI) acid and ethanoic acid.
TEST FOR OXYGEN
When a glowing splint is inserted into a gas jar containing an unknown gas and the glowing splinter is rekindled, then the gas is likely oxygen gas or nitrogen (I) oxide gas.
If the gas is colourless and reacts with nitrogen (II) oxide to produce reddish-brown fumes of nitrogen (IV) oxide, then the gas is confirmed to be oxygen gas.
OXIDES
Oxides are binary compounds formed when oxygen combines with other elements.
Types of oxides (classification)
- Basic Oxides: These are oxides of metals e.g. Na2O, K2O, MgO, CaO etc. They react with acids to form salt and water only. Example:
Na2O(s) + 2HCl(aq) > 2NaCl(s) + H2O(l)
- Acidic Oxides: These are oxides of non-metals which dissolve in water to form acids e.g. CO2, SO2, NO2 etc. They react with alkali to form a salt and water only e.g. CO2(g) + 2NaOH(aq) > Na2CO3(aq) + H2O(l)
- Amphoteric Oxides: These are oxides of metals which can react with both acids and alkalis to form salt and water only. They include the oxides of Al, Zn, Pb and Sn. Example:
ZnO(s) + H2SO4 > ZnSO4(aq) + H2O(l)
ZnO(s) + 2NaOH(aq) + H2O > Na2Zn(OH)4(aq)
- Neutral Oxides: These are oxides of non-metals which are neither acidic nor basic. They are neutral to litmus. They include CO2H2O and N2O
- Peroxides: These are oxides which contain a higher proportion of oxygen than ordinary oxides e.g. Na2O2, CaO2 and BaO2. They react with dilute acid to produce hydrogen peroxide, H2O2
ASSIGNMENT
Why is oxygen collected over mercury?